Fig. 22.1
Indications for surgery for acute epidural hematoma
Volume of hematoma >30 mL regardless of GCS
Midline shift >5 mm
GCS <9
Thickness of hematoma >15 mm
Timing of intervention for acute epidural hematomas:
Surgery <2 h after loss of consciousness improves outcomes
Surgery <70 min after clinical signs of brain herniation improves outcomes
ASAP for GCS <9 with anisocoria
Acute Subdural Hematomas
These injuries frequently involve more serious damage to underlying brain tissues and often have more disability postinjury for that reason.
Indications for decompression of acute subdural hematomas (Fig. 22.2):
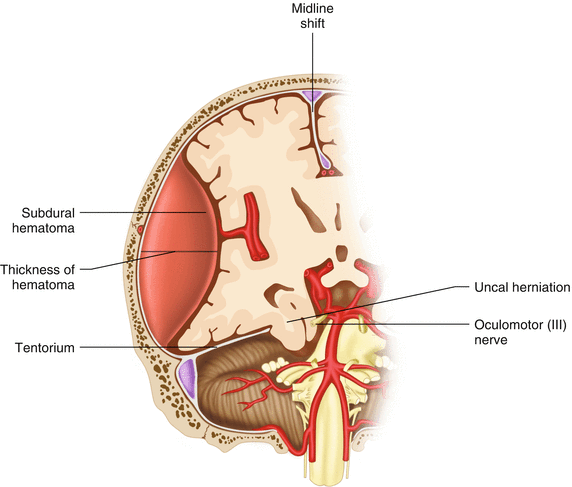

Fig. 22.2
Indications for surgery acute subdural hematoma
Thickness >10 mm
Midline shift >5 mm
Comatose patient with <10 mm thickness or <5 mm midline shift and either ICP >20 mmHg, pupil abnormalities, or deterioration of 2 or more points in GCS
Timing of intervention for acute subdural hematomas:
Stronger correlation between time of onset of coma/deterioration to surgery than of time from injury to surgery
Surgery <2 h after onset of coma/deterioration improves outcomes
Surgery <3 h after clinical signs of herniation improves outcomes
Other Injuries
Indications for decompression of intracerebral hematomas, depressed skull fractures, penetrating injuries, etc.:
Midline shift >5 mm
Pupillary abnormalities
Deterioration of 2 or more points in GCS
Timing of intervention:
Stronger correlation between time of onset of coma/deterioration to surgery than of time from injury to surgery
Surgery <2 h after onset of coma/deterioration improves outcomes
Surgery <3 h after clinical signs of herniation improves outcomes
Preoperative Preparation
Preoperative evaluation and preparation is dependent upon the nature of the patient’s injuries. Multisystem trauma patients should be managed by ATLS priorities to stabilize life-threatening injuries first. Even transient hypoxia or hypotension has a significant negative impact on neurologic outcomes for patients with TBI. Hypotension in multitrauma patients is most likely secondary to extracranial injury and rarely caused by TBI. Control of airway, respirations, and hemorrhage take priority over cranial decompression and should be dealt with first.
Patients requiring decompressive craniotomy should be treated with Mannitol (dose of 0.5–1.0 g/kg) preoperatively to help reduce intracranial pressure (ICP) and improve cerebral perfusion pressure. Other measures to reduce ICP include modest hyperventilation (only as a temporizing measure prior to surgery), hypertonic saline, and diuretics. The use of steroids is not indicated. Prophylactic antibiotics (usually Cefazolin) are used in our practice. As these patients are not typically mobile, they do have risk of venous thromboembolism (VTE). Mechanical VTE prophylaxis with sequential compression devices is certainly warranted. Pharmacologic VTE prophylaxis can be considered postoperatively on an individual case basis. The scalp is clipped with electric clippers immediately prior to surgery and skin preparation.
Patient positioning is important as well. Injury to a dural sinus (either from the trauma or intraoperative) can occur. In that event, raising the head as much as possible (reverse trendelenberg and/or sitting position) can significantly reduce the volume of bleeding. Angulation of the neck with resultant compression of the jugular veins can also increase venous backpressure. We prefer to have the ability to adjust the head position during the procedure (with cervical spine precautions) so we use a gel doughnut to hold the head. If the spine has been cleared radiologically, then position the patient’s hips to allow a sitting position if needed. If the spine has not been cleared, then the patient must remain level from the hips up by flexing the bed at the hips. In the absence of clearance of the cervical spine (and associated abdominal or thoracic injuries requiring surgery) the patient may be placed in a lateral position to protect the cervical spine.
Operative Strategy
The principal goal of decompressive craniotomy is to reduce ICP and improve cerebral perfusion by adequate decompression of the brain and control of ongoing bleeding to prevent recurrence. This requires adequate exposure in order to accomplish these goals.
Secondary goals (but also of prime importance) are to prevent further progression of brain injury and secondary injury due to operative trauma.
Operative Technique
After induction of anesthesia, appropriate positioning, skin prep, and draping, a question mark incision is marked just anterior to the tragus. It is also a good idea to mark the midline to help avoid injury to the sagittal sinus. The incision should start at the level of the zygoma. The incision should be carried through the skin, galea, and down to the bone. It should be extended well posterior to the ear then curve a few centimeters lateral to the sagittal suture and continue forward to the hairline. The superficial temporal artery should be preserved if possible to improve blood supply to the flap. Bleeding from the skin incision (which can be significant) can be controlled by various means. Most common is the use of Raney clips (if available). If you have these, the incision can be made in segments and the clips applied to each segment before continuing the incision. More likely in a rural setting, you will not have access to these. We use the cautery (with coagulation set to 50) and have been able to achieve excellent hemostasis with this (Fig. 22.3a).