CHAPTER 29 Decompression Surgery
Description
Terminology and Subtypes
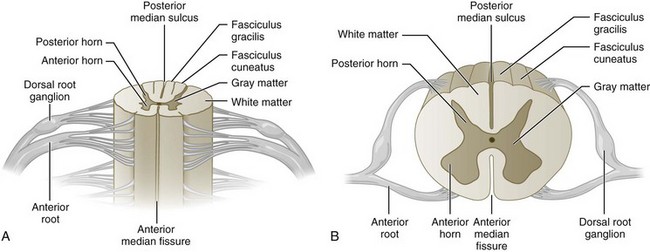
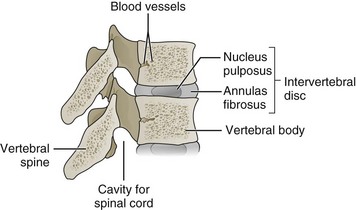
Decompression surgery refers to the various forms of lumbar surgery whose primary goal is to remove structures in the neural canal that are thought to cause neural impingement.1,2 Neural impingement is a term used to describe both sensory (e.g., pain, numbness, tingling, burning) and motor (e.g., reduced strength, muscle atrophy) changes that may result from a compression of structures within the neural canal. In the cervical and thoracic spine, the neural canal contains the spinal cord, from which anterior and dorsal roots emerge to form spinal nerve roots (Figure 29-1). In the lumbar spine, the neural canal contains the cauda equina, composed of lumbar and sacral spinal nerve roots (Figure 29-2). The borders of the neural canal include the vertebral body, intervertebral disc, pedicles, lamina, and zygapophysial joints (also called facet joints), as well as the longitudinal ligament and the ligamentum flavum. The lateral recess is defined by the medial border of the superior facet and the medial border of the pedicle (Figure 29-3). This area, also called the subarticular space, is a common location for nerve root impingement within the neural canal (Figure 29-4). The term degenerative cascade is used to describe changes in the discs, facet joints, and ligamentum flavum that may eventually lead to neural impingement.3 Disc herniations are often described according to the direction in which the bulge occurs, that is, posterolateral, central, or far lateral. Spinal stenosis refers to the narrowing of the neural canal, which often results from advanced degenerative changes in surrounding structures.4
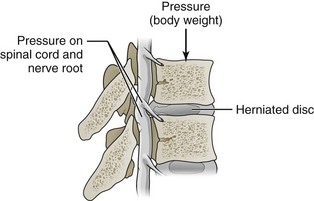
Figure 29-4 The lateral recess is a common location for spinal nerve root impingement secondary to disc herniations.
(Modified from Patton KT, Thibodeau GA. Anatomy & physiology, ed. 7. St. Louis, 2010, Elsevier.)
Symptoms associated with neural canal impingement in the lumbosacral region may include radiculopathy and neurogenic claudication.2 Radiculopathy is a general term used to describe symptoms involving a spinal nerve root, both sensory and motor. Sciatica is the term given to radiculopathy of the sciatic nerve. Claudication refers to intermittent pain in the legs that is often worse after activities such as walking or standing and is relieved only by sitting or leaning forward. Neurogenic claudication describes claudication from neural canal impingement, usually from spinal stenosis. Cauda equina syndrome represents a medical emergency in which severe neural impingement affects multiple lumbar or sacral spinal nerve roots. Signs and symptoms of cauda equina syndrome may include bowel and bladder dysfunction (e.g., incontinence, inability to urinate), saddle anesthesia, or progressive leg weakness.
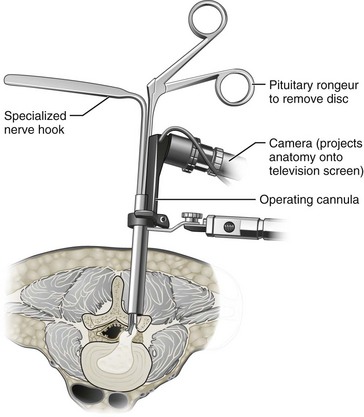
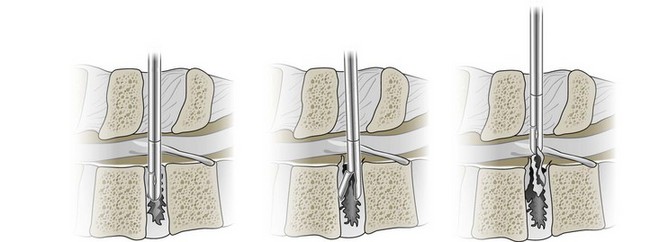
There are many types of decompression surgery, which are usually named according to the structure that is surgically removed. The two main types of decompression surgery are discectomy and laminectomy. Discectomy implies surgical excision of an intervertebral disc, whereas laminectomy involves removal of the lamina. Either procedure may involve partial or complete removal of the targeted structures. There are several variants of decompression surgery procedures according to the surgical technique used. Standard, traditional, or open decompression usually describes a procedure in which the skin incision made is large enough to completely visualize the targeted structures. Microscopic decompression is a loosely defined term in which the skin incision made is typically smaller and different surgical instruments are used to improve visualization of the targeted structures with less tissue disruption (Figure 29-5). An endoscope or arthroscrope may be used in microscopic decompression to improve visualization of deeper structures. The procedures and techniques used in decompression surgery are often combined to name a particular approach (e.g., microscopic discectomy, microdiscectomy, or endoscopic discectomy). Multiple decompression procedures may also be combined (e.g., discectomy with or without laminectomy).
History and Frequency of Use
The earliest evidence of spinal pathology can be traced back to the preserved remnants of Egyptian mummies approximately 5000 years ago.5 The first written account of spinal pathology originated in 1600-1700 bc in a surgical textbook from Egypt that was later transcribed as the Edwin Smith Papyrus.6,7 In more modern times, Andreas Vesalius was one of the first to raise awareness about the intervertebral disc in his publication De Humani Corporis Fabrica.8 However, it was only in the past two centuries that decompression surgery was attempted to address specific spinal pathology.
In 1829, Smith reported performing a lumbar laminectomy in the United States for the treatment of progressive paresis attributed to a vertebral fracture.9 Lumbar laminectomy for the treatment of spinal stenosis followed several decades later when the procedure was performed by Lane in 1893.10 One of the earliest accounts of discectomy is attributed to Oppenheim and Krause, who performed a laminectomy followed by transdural disc resection in 1909.11,12 In 1910, a unilateral laminectomy was performed in cadavers by Taylor.12 Shortly thereafter, there were published accounts of a herniated disc.13,14 The term neurogenic claudication was also coined around that time by Dejerine, although the link with lumbar spinal stenosis was not made until 1954 by Verbiest.15,16
In 1929, Dandy operated on two individuals presenting with compression of the cauda equina following traumatic disc herniation.17 The now seminal report by Mixter and Barr in 1934 was the first to correlate sciatica with a herniated lumbar disc and elaborate on its operative management with a laminectomy and discectomy.18 The procedure for decompression surgery remained largely unchanged until microsurgical techniques were developed in the early 1970s to improve outcomes and minimize adverse events (AEs).19–21 In 1978, Williams reported on 532 patients who received lumbar microdiscectomy through an intralaminar window.22 A decade later, Young and colleagues described a unilateral approach for bilateral spinal canal decompression.23 In the 1990s, development of tubular retractor systems and low-profile automatic instrumentation revolutionized decompression surgery through smaller incisions, less operative blood loss, increased visualization of pathology, decreased hospitalization, shorter postoperative recovery, and earlier return to activities.
The United States has the highest rate of spine surgery in the world.24–28 Lumbar discectomy is the most common surgical procedure performed in the United States for patients presenting with chronic low back pain (CLBP) and radiculopathy due to neural impingement.29–31 Spinal stenosis is the most common indication for lumbar spine decompression surgery in patients older than 65 years of age.32,33 Between 1979 and 1992, surgical treatment for spinal stenosis increased in the United States by almost 700%, from 8 to 61 procedures per 100,000 persons.34 Between 1992 and 2003, the rate of lumbar discectomy and laminectomy in the United States increased from 1.7 to 2.1 per 1000 Medicare enrollees, resulting in nearly 300,000 procedures annually in that population.29,35,36
Practitioner, Setting, and Availability
According to the American Academy of Orthopaedic Surgeons (AAOS), there were 17,673 orthopedic surgeons and 4605 residents practicing in the United States as of January 2010.37 Corresponding figures from the American Association of Neurological Surgeons (AANS) were 3519 neurologic surgeons and 1663 residents/fellows as of December 2009.38 The number of orthopedic and neurologic surgeons with subspecialty training in spine surgery is difficult to estimate because board certification in spine surgery is optional in the United States. The American Board of Spine Surgery (ABSS) was approved as a specialty certification board by the Medical Board of California in 2002 and reported 180 board-certified spine surgeons in the United States as of January 2010 (personal communication, Eckert M. Executive administrator, American Board of Spine Surgery, 2010).
Procedure
Intervertebral Disc
Patients with sciatica related to large disc herniations usually require surgical removal of the prolapsed portion of the disc. This can be accomplished through a unilateral approach with only minimal resection of the posterior elements as described above to identify the spinal nerve root. Once exposed, the spinal nerve root is then mobilized and protected prior to exposing the prolapsed section of the disc to be removed. The amount of intervertebral disc removal varies considerably, and debate continues over removal of disc fragments contained within the annulus (Figure 29-6).
Theory
Mechanism of Action
Kirkaldy-Willis and colleagues described a degenerative cascade involving the intervertebral discs, facet joints, and ligamentum flavum that could eventually lead to neural impingement if severe.3 This degenerative cascade initially involves dehydration of the disc and loss of disc height that naturally occurs with aging. These changes in disc composition and morphology may then lead to posterior bulging, which exerts pressure on the ligamentum flavum and pushes it toward the neural canal. Further degeneration or injury to a degenerated disc may then lead to tears in the annulus, resulting in prolapse of the nucleus pulposus into the neural canal.
Impingement of the neural canal may result in neurologic dysfunction from direct or indirect causes. Spinal nerve root function may be disrupted due to direct mechanical compression, though the amount of pressure required to do this remains a matter of continued research. An alternative mechanism that has been proposed is that spinal nerve root function may be affected indirectly by chemical mediators of the acute inflammatory cascade (e.g., cytokines).39 Claudication may result from direct mechanical compression of spinal nerve roots or from focal ischemia.39
Indication
As described above, the primary goal of decompression surgery is to remove structures that may be contributing to neural impingement and any resulting neurologic dysfunction from the spinal nerve roots.2 The indication for decompression surgery in the context of CLBP is persistent and disabling pain or neurological dysfunction associated with impingement of the neural canal. Radicular and claudicant leg pain are the typical pain syndromes associated with impingement of the neural canal. Specific diagnoses that may cause impingement of the neural canal include spinal stenosis, lateral foramen impingement, and large disc herniations. Because of the potential harms involved with decompression surgery, it should only be considered when patients have failed to achieve meaningful improvement of their CLBP and neurologic dysfunction following appropriate nonoperative management, since many patients will improve without treatment given sufficient time.40,41
Assessment
Before undergoing decompression surgery, patients should first be assessed for LBP using an evidence-based and goal-oriented approach focused on the patient history and neurologic examination, as discussed in Chapter 3. Clinicians should also inquire about medication history to note prior hypersensitivity/allergy or AEs with drugs similar to those being considered, and evaluate contraindications for these types of drugs. Advanced imaging such as magnetic resonance imaging (MRI), computed tomography (CT), or CT myelography is required to help guide the spine specialist to target the appropriate spinal levels involved in a patient’s CLBP and related neurologic dysfunction.
Another aspect that should be considered for the assessment of patients with CLBP who may be candidates for decompression surgery is to clearly identify the location of predominant pain symptoms. It has been suggested that decompression surgery is most beneficial for patients whose CLBP is associated with predominant leg pain rather than predominant axial LBP. Because symptom severity is expected to fluctuate over time and the wording of a question may influence its answer, establishing the location of the predominant pain may require a validated instrument. Responses to a three-item questionnaire developed for this purpose was as useful as assessment by a pain management physician or advanced imaging findings in determining the likelihood that patients presenting with CLBP were deemed appropriate candidates for decompression surgery by a spine surgeon.42
Efficacy
Clinical Practice Guidelines
Discectomy
The CPG from Belgium in 2006 found no evidence supporting discectomy among patients with disc prolapse without neurologic involvement.43 That CPG also reported low-quality evidence supporting discectomy among patients with discoradicular conflict and neurologic involvement.
The CPG from Italy in 2006 recommended discectomy for LBP with neurologic involvement.44
The CPG from Europe in 2006 did not recommend decompression surgery in general for CLBP.45
Laminectomy
Findings from the above CPGs are summarized in Table 29-1.
TABLE 29-1 Clinical Practice Guideline Recommendations on Decompression Surgery for Chronic Low Back Pain
| Reference | Country | Conclusion |
|---|---|---|
| 43 | Belgium | Not recommended for disc prolapse without neurologic involvement Low-quality evidence for discoradicular conflict with neurologic involvement |
| 45 | Europe | Not recommended |
| 131 | Italy | Recommended for LBP with neurologic involvement |
LBP, low back pain.
Systematic Reviews
Discectomy
Cochrane Collaboration
The Cochrane Collaboration conducted an SR in 2007 on surgery for degenerative lumbar disc prolapse.46 A total of 42 RCTs were identified, of which 22 involved some form of discectomy. Sixteen of these RCTs examined disc herniation,47–62 one examined nerve root compromise,63 one examined root lesion,64 two examined nerve root pain with or without LBP,65,66 one examined disc prolapse,67 and one examined a mixture of patients without specifying the duration of LBP or condition.68
One RCT found that discectomy was superior to conservative treatment after 1 year yet statistically significant differences were not observed after 4 and 10 years of follow-up. Another RCT observed similar findings for microdiscectomy versus physical therapy.51 A meta-analysis of two RCTs showed no statistically significant differences between discectomy and chymopapain injection for subjective outcomes, which was consistent with another meta-analysis of three RCTs reporting on the surgeon’s rating of patient improvement.49,55,59
However, another meta-analysis showed that discectomy was superior to chymopapain injection regarding the need for a second procedure within 1 to 2 years.49,50,59,65 Yet another meta-analysis of two RCTs indicated that chymopapain injection was superior to automated percutaneous discectomy regarding the need for a second procedure within 1 year.53,57 RCTs of microdiscectomy versus open discectomy did not show any differences.54,58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree