Fig. 15.1
Damage control sequence. Part 2 occurs in the ICU. Parts 1 and 2 may be repeated multiple times over several days to a week prior to Part 3 definitive repair
Indications for Damage Control Surgery
The goal of damage control surgery is to recognize patients who are physiologically deranged, need second explorations, or are at risk for complications if the traditional approach with closure is undertaken. The lethal triad of hypothermia, coagulopathy, and acidosis appears as the patient reaches physiologic exhaustion, so waiting for the triad to develop and then undertaking damage control defeats the purpose of damage control. Bleeding and contamination are controlled in the first operation. Then, the patient is taken to the intensive care unit (ICU) for resuscitation, allowing time to recapture the patient’s physiology.
Identification of patients who benefit from damage control surgery is an art that requires experience and communication. Emergency medical services (EMS) can communicate valuable information prior to patient arrival, such as prehospital hypotension, hypothermia, blood loss, and ongoing hemorrhage that can trigger the trauma team to entertain damage control. Even a single episode of prehospital hypotension that resolves with resuscitation can be indicative of a severely injured patient with little reserve for a lengthy operation [12]. Intraoperatively, hypothermia less than 36 °C, an acidosis less than 7.25 or base deficit greater than 8, clinical coagulopathy or based on laboratory values with an international normalized ratio (INR) greater than 1.5 or fibrinogen <200 mg/dl (<2 g/l) are indications for damage control. Constant and effective communication with anesthesia is necessary to ensure frequent monitoring, guide resuscitation, and communicate the decision to abort the operation and rapidly proceed to the ICU.
Patients with multiple cavity injuries are ideal candidates for damage control. Ongoing bleeding can hasten physiologic exhaustion, so hemorrhage control across cavities must be expeditiously treated with no opportunity for definitive repair. For example, a patient with a thoracoabdominal injury or multiple stab wounds may need both the abdomen and mediastinum or thorax explored, and the surgeon must make a judgment about which cavity is the primary source of bleeding or life-threatening injury. Once bleeding is controlled in one cavity, the surgeon must rapidly examine the next. Other situations that lend themselves to damage control are those where endovascular techniques may achieve hemorrhage control more effectively such as severe liver or pelvic bleeding. While waiting for the endovascular team to arrive, the surgeon may explore the abdomen and pack the liver or pelvis and even isolate and temporarily occlude the porta hepatis or internal iliac arteries. Once the endovascular team is available, the surgeon and radiologists can work together to combine operative and endovascular interventions to stop bleeding. Patient selection also plays a role; the elderly, those with more comorbidities, and pediatric patients have less reserve, and thus, the team should have a lower threshold for damage control. Ultimately, the earlier the decision is made to undertake damage control, the better chance of salvaging the patient.
Ground Zero: Scene to Emergency Department
Advanced Trauma Life Support (ATLS) is the backbone of prehospital treatment. Transport to a definitive trauma center without delay is the primary goal of ATLS and prehospital care with a goal of less than 30 min from call initiation to arrival at the trauma center. An airway must be established if a patient cannot protect his own. Needle decompression or tube thoracostomy may be performed for hypoxia and loss of breath sounds. Large-bore IVs should be placed, and resuscitation begun with isotonic crystalloid. Hemorrhage can be controlled with tourniquets or digital pressure. Fractures can be splinted to provide stability and decrease ongoing bleeding. Previously, 2 l of isotonic crystalloid were given followed by either more crystalloid or blood products if available to achieve a desired response in vital signs. As discussed in Chap. 14, under certain circumstances, a systolic blood pressure of 80–90 mmHg may be more ideal until hemorrhage is controlled [13]. This practice of “permissive hypotension” primarily applies to penetrating trauma with a short anticipated transport time to definitive care and only in the absence of head injuries and significant patient comorbidity.
Frequent, effective communication is imperative between the prehospital and emergency department teams. Updates on vital signs and physical findings allow emergency department personnel to mobilize resources. Necessary equipment can be gathered and procedure trays opened. Radiology technicians can be at the bedside waiting with portable X-rays and can expedite any other radiological interventions such as computed tomography (CT). The blood bank can be notified if a massive transfusion is planned in order to begin thawing products. Most importantly, roles during the triage are assigned and performed in an organized manner. Mobilization of the team prior to patient arrival decreases evaluation time and eliminates delay to imaging or the operating room.
The patient should ideally spend as little time as possible— certainly no more than 20 min—in the emergency department resuscitation/trauma area including procedures and adjuncts (Fig. 15.2). The trauma team must decipher what the life-threatening injuries are which determines the next stage of damage control. In trauma patients with blunt mechanisms, multiple cavities may be involved, and the sources of hemorrhage difficult to identify as they may not be visible. Penetrating traumas are much easier to triage, given the external wound. It is important to determine trajectory; the external wound may appear to lie within a single cavity, but trauma may involve multiple cavities. It is important to place a marker such as a paperclip or electrocardiogram (EKG) lead on the external wound prior to imaging in order to approximate trajectory.
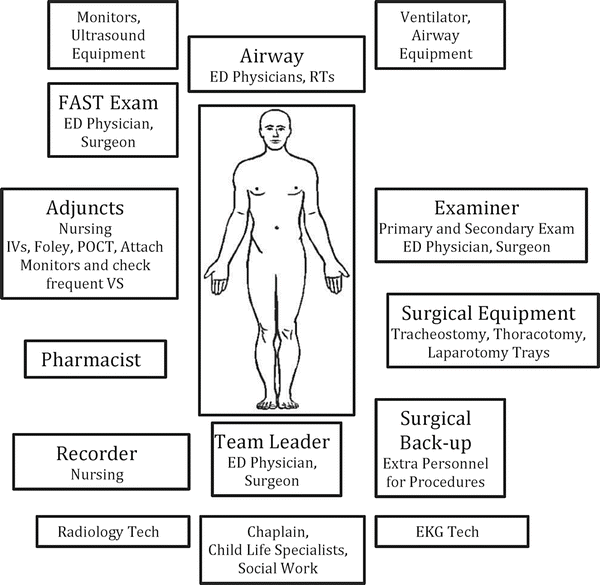

Fig. 15.2
Arrangement of Emergency Department resuscitation area conducive to effective communication. Note that the Recorder is adjacent to the Team Leader to read back information. Examiner should be on patient’s left side to facilitate Emergency Department (ED) thoracotomy and other surgical procedures if necessary. Supply carts and medication dispensers/storage should be in close proximity if not in the same room along the walls. RT = Respiratory Therapist, POCT = Point of Care Testing, VS = Vital Signs, EKG = Electrocardiogram
The majority of trauma patients who are hypotensive are in hemorrhagic shock. A patient may exsanguinate externally or internally (thorax, abdomen, pelvis, retroperitoneum, soft tissues). If life-threatening bleeding is ongoing in one of the above mentioned cavities and/or the patient unstable, the surgeon should proceed rapidly to the operating room. Should a patient arrest just prior to arrival or in the resuscitation bay, an emergent resuscitative thoracotomy may be performed to release a cardiac tamponade and/or occlude the aorta in order to maintain perfusion to the heart and brain. Since endovascular technology has further evolved, the use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in trauma is being revisited [14]. While it cannot relieve a cardiac tamponade, REBOA can be used in blunt or penetrating trauma prior to arrest to manage non-compressible hemorrhage at multiple levels of the aorta without the morbidity of a large chest wound [15]. The femoral artery may be accessed percutaneously or by cut down, and balloon placement does not require fluoroscopy [14, 15].
Depending on patient stability and resource availability, the team may elect to obtain a CT to gain further information. If a liver injury or pelvic fracture with bleeding is found, the team may proceed to a hybrid operating and endovascular room (when available) to control hemorrhage operatively while mobilizing the endovascular team.
Again, effective communication is of utmost importance in efficient patient flow. The CT technologist should be notified that the patient will be arriving imminently. The ordered scans should be discussed and clarified. It helps the technologist and radiologist reading the imaging to know the history (including mechanism) and physical exam findings as well as the suspected injuries as they may recommend arterial and venous phased scans, thinner slices through worrisome areas, or additional scans while the patient is still on the table. If there is a possibility the patient may be proceeding to the operating room, notifying the operating room team at the earliest opportunity is ideal. Some centers place the operating room (OR) staff on standby when the trauma team is activated in the emergency department. While a trauma-ready operating room is always available at a Level 1 center, the lights can be turned on, the room and bed warmed, and the nurse, scrub technician, and anesthesia team mobilized to prepare for a case. A trauma cart with basic supplies (shunts, staplers, tubes, drains, vacuum dressings) and various trays (vascular, thoracotomy, laparotomy) as well as a trauma suture tree should already be available in the room or just outside. Finally, the massive transfusion protocol should be implemented as soon as deemed necessary to ensure products are available as soon as possible whether it be in the ICU or operating room.
Damage Control Part 1: Operative Intervention
There are two goals in damage control Part 1: control of bleeding and contamination. Upon arrival to the room, the surgeon may give the team a brief history, interventions undertaken thus far, lines and tubes in place or needed, and the overall plan for the operation. It can be extremely helpful if anticipated problems are vocalized, so that anesthesia staff can prepare for the resuscitation and have rapid transfusers and cell savers available, while the OR staff can ready an abundant supply of sponges, basins, and adequate suction. In extreme situations, intubation may be occurring while prepping and draping the patient. In some instances, time will only permit splash prep.
Positioning the patient is dependent on which cavities or extremities need to be explored as previously determined in the emergency department. Any extremity may be prepped, draped, and included in the operative field. If a vascular injury is suspected, both legs from the inguinal ligament to knees should be prepped in case vein graft is needed. Generally, the trauma patient is supine with both arms abducted at 90° and prepped from chin to knees and laterally to the bed. If a combined thoracotomy and laparotomy is entertained and the hemithorax previously determined, a modified taxi cab hailing position is ideal. The patient is primarily supine, but on the ipsilateral side of the thorax to be entered, the chest wall is rotated medially about 30° to the coronal plane and supported with a roll. The ipsilateral arm is abducted at 90° and elbow flexed at 30°.
Once a cavity is opened, hematoma and blood should be evacuated (usually manually) and the cavity packed with lap sponges. If exsanguination is temporized, anesthesia should be allowed to aggressively resuscitate the patient until bleeding restarts or until the systolic blood pressure is 80–90 mmHg. All injuries must be fully exposed to localize hemorrhage and contamination. Bleeding organs on a pedicle (spleen, kidney) should ideally be sacrificed. Liver and lung resections are non-anatomical and usually performed with staplers. Finger occlusion of a pedicle and the Pringle maneuver for the liver or twisting the lung at its hilum are fast techniques to control significant bleeding. Various maneuvers (Kocher, Mattox, Cattell-Braasch) expose the retroperitoneum. Most vessels may be ligated. If a vessel supplies an end organ or extremity, the vessel should be shunted [16–19]. However, in life-threatening situations, even the inferior vena cava may be ligated at its bifurcation. Visceral contamination is controlled by stapling and removing the injured segment of bowel or simply whip stitching the injury closed. If a segment is removed, the patient is left in discontinuity due to time and the need for a second look given the possibility of further necrosis. A temporary abdominal, chest, or extremity dressing is placed, allowing for rapid reentry or examination while preserving the fascia and skin for definitive closure. These may be manufactured or homemade with negative pressure applied. Incorrect counts are common due to the emergent nature of the operation. While attempts are made to count the number of sponges and instruments left in a packed, open cavity, the count should never delay placement of a temporary dressing and transport to the ICU.
Again, communication with bed control to ensure an ICU bed is available and with the ICU nurses and physicians eases the transition to the next stage of damage control. It may take time to move another patient out of an ICU room, clean the room, and bring the hospital bed to the operating room. Report can be called about 20–30 min prior to leaving the operating room which allows the ICU staff time to set up suctioning, warming, and massive transfusion equipment, gather pumps, tubing and supplies, and prepare for the patient as well as notify respiratory therapy to bring a ventilator to the ICU room.
Damage Control Part 2: Resuscitation
The goal of Part 2 is to continue aggressive resuscitation in a rapid fashion in order to correct the physiologic derangements. Upon arrival to the ICU, the surgical team should communicate the brief history, interventions, the definitive plan, and any specific concerns. A full laboratory panel should be sent upon arrival to the ICU including a complete blood count (CBC) with differential, complete metabolic panel (CMP) with all electrolytes, creatine kinase (CK), lactic acid (LA), arterial blood gas (ABG), and coagulation panel including fibrinogen and repeated at minimum every 4–6 h (up to every 1–2 h in certain circumstances) to guide resuscitation and organ perfusion endpoints. Serial troponins and electrocardiograms may also be included. Core temperature should be monitored and rewarming measures such as blankets and warmed fluids used because hypothermia can inactivate the clotting cascade and impede the body’s ability to coagulate blood.
While the resuscitation ratio is debated, a 1:1 or 1:2 ratio of packed red blood cells (pRBCs) to fresh frozen plasma (FFP) is the current recommendation. The goal of resuscitation is to achieve a hemoglobin ≥ 7 g/dL (>70 g/l) (>9 g/dl, 90 g/l in an actively bleeding patient), INR <1.5, maintain platelets >100,000, and cryoprecipitate may need to be given if the fibrinogen is <200 mg/dl (<2 g/l). If these goals are met, isotonic crystalloid may be used, but be mindful that normal saline may lead to a non-anion gas metabolic acidosis, worsening coagulopathy.
There is no single resuscitative endpoint. Clinically, urine output may be measured and stabilization in vital signs with titration of pressors off is indicative that end-organ perfusion is being achieved. The characteristic of the output from the temporary vacuum dressing and the amounts from the drains and tubes should be monitored. Ultrasound can help guide resuscitation, as intravascular volume can be based on inferior vena cava (IVC) collapsibility and cardiac contraction. This will be discussed further in Chap. 22. Corrections of the coagulopathy, hypothermia, and acidosis are guidance parameters.
Another important role of the ICU provider is to perform a thorough tertiary survey including physical examination and review of pertinent imaging and blood work to ensure that no injuries or wounds have been missed. Once resuscitation endpoints are met ideally within 24–36 h, the patient is returned to the operating room for a second look, or Part 3—definitive repair. If at any point during Part 2 the acidosis or coagulopathy is not correcting or was trending in the correct direction, but then regresses, or if there is clinical evidence of ongoing, rapid hemorrhage, the patient should be immediately returned to the operating room as this is indicative of a missed injury or ongoing, uncontrolled bleeding.
Finally, complications of resuscitation can arise. Acute respiratory distress syndrome (ARDS) and transfusion-related acute lung injury (TRALI) can result from aggressive resuscitation and blood product administration. One should, however, consider other differential causes for persistent hypoxemia, i.e., abdominal compartment syndrome. In the event of persistent hypoxemia, lung protective strategies such as ARDSNet ventilation should be implemented.
Compartment syndrome may develop in the abdomen even with a temporary dressing in place. It should be suspected if cardiac return is low, the IVC is collapsed on ultrasound, and the urine output decreases when previously appropriate or in the event of persistent hypoxia or hypercarbia with climbing ventilation pressures. Bladder pressures should be measured frequently or even continuously. If pressures remain high, the dressing may need to be modified, loosened, or reapplied.
For extremities, a Stryker needle can be used to objectively quantify the pressure; rapid, significant increases in compartment pressures, a measured compartment pressure >30 mmHg, or <30 mmHg difference in the diastolic blood pressure and measured compartment pressure should prompt fasciotomies.
Damage Control Part 3: Definitive Repair
Once the patient is resuscitated as defined by meeting end-organ and hemodynamic endpoints, the patient is returned to the operating room for definitive repair. The temporary dressing and all packs are removed. The cavity should be thoroughly explored. If at any point the patient becomes hemodynamically unstable or physiologically deranged as in Part 1, begins re-bleeding, or demonstrates they are unable to undergo a lengthy operation, the temporary dressing may be reapplied and the patient returned to the ICU for further resuscitation. Definitive repair entails restoring bowel continuity, tissue debridement, and vascular grafts and anastomoses. Prior to closing the abdomen, an X-ray should be obtained and confirmed with radiology that no foreign bodies remain in the cavity. If multiple cavities are left open in Part 1, all cavities may be closed in Part 3 or only one and Part 3 repeated for each cavity.
Damage Control Strategy Under Special Circumstances
The following represents specific treatment strategies for unique conditions. The ultimate goal of each strategy is to implement the damage control concept early in care, combat the lethal triad, and transport victims safely for definitive management.
Blast Injuries
Blast injuries are challenging as patients can suffer from both penetrating and blunt mechanisms. Treatment goals remain the same, and ABCs initially assessed. The provider should not become distracted by the often unsightly injury, but rather focus on treatment according to protocol and standard practice. Cricothyroidotomy may be necessary with a blast to the face. Damage control with the blast-injured patients is done in large part by controlling hemorrhage. Hemorrhage sites are either anatomically compressible (e.g., extremity, or axillary/groin vascular injuries) or completely non-compressible (e.g., truncal injuries). Patients with non-compressible hemorrhage sources receive the highest priority for immediate transport to a hospital. Compressible hemorrhage sites are amenable to direct digital pressure or tourniquet control, which can be instituted by first responders. Control of bleeding with proximally arterial compression is not advised as it does not address venous hemorrhage. Using large stacks of gauze or additional dressings in lieu of manual compression should be avoided, as this technique dissipates the pressure applied directly to the bleeding site and may delay identification of ongoing bleeding [20].
While use of tourniquets has been controversial in the damage control situation, multiple reports in the literature of tourniquet use have defined their advantages [21–26]. These include improved hemorrhage control upon patient arrival, decreased incidence of shock in those casualties treated with tourniquets, improved survival, and acceptably low tourniquet-related complications. Tourniquets should be applied to exsanguinating extremities as soon as possible in damage control situations. It is generally recommended that restoration of arterial blood supply must be completed within 6 h from placement of the tourniquet [20]. Prior to patient arrival, it is helpful for the emergency department personnel to know if a tourniquet was placed and when, the characteristic of bleeding (dark non-pulsatile versus bright red, pulsatile), and a description of the injuries. When giving report at patient arrival, the transport team should include the time of injury and approximate amount of blood loss at the scene.
If the patient’s bleeding is controlled upon arrival, the primary and secondary surveys should be rapidly conducted in the usual fashion, and the four remaining cavities assessed for hemorrhage with the usual adjuncts. Blast injuries can create penetrating wounds from shrapnel, but can throw a patient with great force, causing blunt injuries as well. This is the ideal situation for damage control. When proceeding to the operating room, the staff should be told to obtain a sterile pneumatic tourniquet and prepare for abdominal and extremity exploration and temporary dressings. If extremity hemorrhage is controlled with a tourniquet and the patient’s FAST is positive and if two teams are available, both the extremity and abdomen may be explored concurrently; in the case of a single operative team, however, one should begin with abdominal exploration if the extremity hemorrhage is controlled with a tourniquet. All exsanguination must be expeditiously stopped.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





