CHAPTER 79
Cryoneuroablation
INTRODUCTION
Cryoneuroablation is an interventional technique aimed at temporary destruction of the nerve providing relief of the pain. The origin of this technique lies in the ancient time but modern cryoanalgesia traces its roots to Cooper et al.1 In 1962, they developed a device that used liquid nitrogen circulating through a hollow tube, insulated except at the tip, to achieve a tip temperature of –196,°C. Amoils,2 an ophthalmic surgeon, developed a simpler handheld device in 1967, which used high-pressure carbon dioxide or nitrous oxide and could achieve temperatures of –70°C. Lloyd et al3 coined the term “cryoanalgesia” for its use in pain management. They proposed that this technique was superior to other methods of peripheral nerve destruction, for example, alcohol, phenol, or surgical lesions, because it is not followed by neuritis or neuralgia. The cryoneuroablation unit:
• The cryoneuroablation relies on the ability of specially constructed probe to freeze tissue around it.
• The cryoprobe consists of a hollow tube with a smaller inner tube.
• Pressurized gas (usually N2O or CO2) at 600 to 800 psi travels down the inner tube and is released into the larger outer tube (which is at a low pressure of 10-15 psi) through a very fine aperture (0.002 mm), which allows the gas to rapidly expand into the distal tip (Figure 79-1).
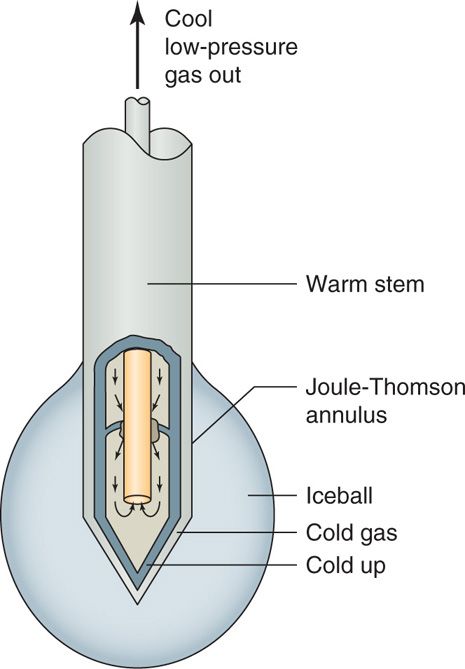
Figure 79-1. Cryoneuroablation probe physics. (Reproduced with permission from Epimed, Farmers Branch, TX.)
• The heat is extracted from the tip of the probe, resulting in temperatures as cold as –89°C at the tip itself (Joule-Thompson effect), forming an ice ball with temperatures in the range of –70°C (Figure 79-2).
Figure 79-2. Cryoneuroablation ice ball. (Reproduced with permission from Epimed, Farmers Branch, TX.)
• The gas is then vented back to the machine itself through the outer tube and is scavenged through a ventilated outlet.
• The “closed system” construction of the probe and machine ensures that no gas escapes into the patient’s tissues.
• Precise gas flows are necessary for safe and effective cryoneuroablation; inadequate gas flows will not produce an ice ball, while excessive flows can cause freezing proximally up the probe, which may increase the risk of skin burns.
• The probe includes a built-in nerve stimulator with sensory and motor capabilities, which allows precise localization of the target nerve (Figure 79-3).

Figure 79-3. Cryoneuroablation machine. (Used with permission from Andrea Trescot, MD.)
The mechanism of cryoneurolysis:
• Thermal injury interrupts the neuronal pathway and provides pain relief. More specifically, the application of cold to tissues creates a conduction block, similar to the effect of local anesthetics.
• At 10°C, larger myelinated fibers stop conducting, but all nerve fibers stop conducting at –20°C.
• The extent and duration of the effect is therefore a function of the degree of cold obtained and the length of time of exposure.
• Long-term pain relief from nerve freezing occurs because ice crystals create vascular damage to the vasa nervorum, which produces severe endoneural edema. This creates a Wallerian degeneration but leaves the myelin sheath and endoneurium intact (Figure 79-4).
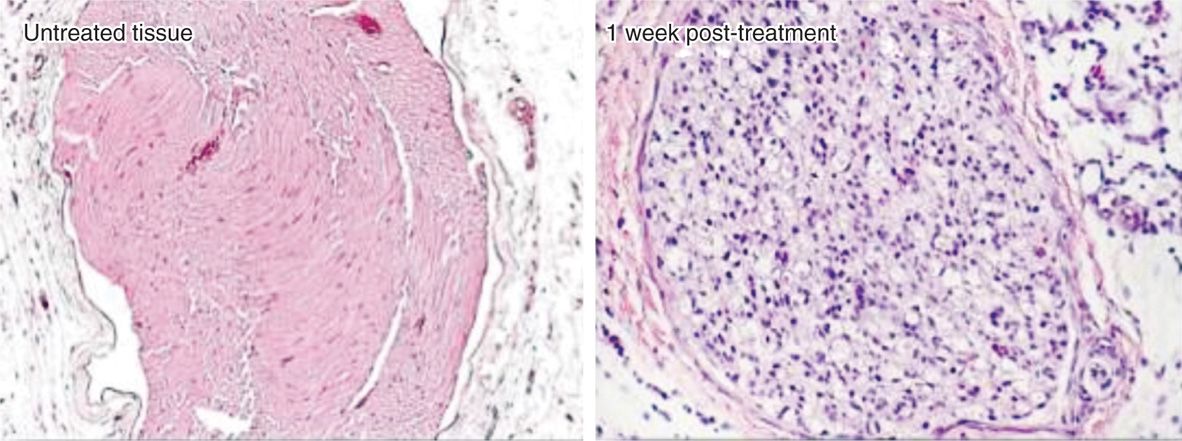
Figure 79-4. Histology after cryoneuroablation. (Reproduced with permission from Myoscience, Redwood City, CA.)
• The Schwann cell basal lamina is spared and ultimately provides the structure for regeneration (Figure 79-5)
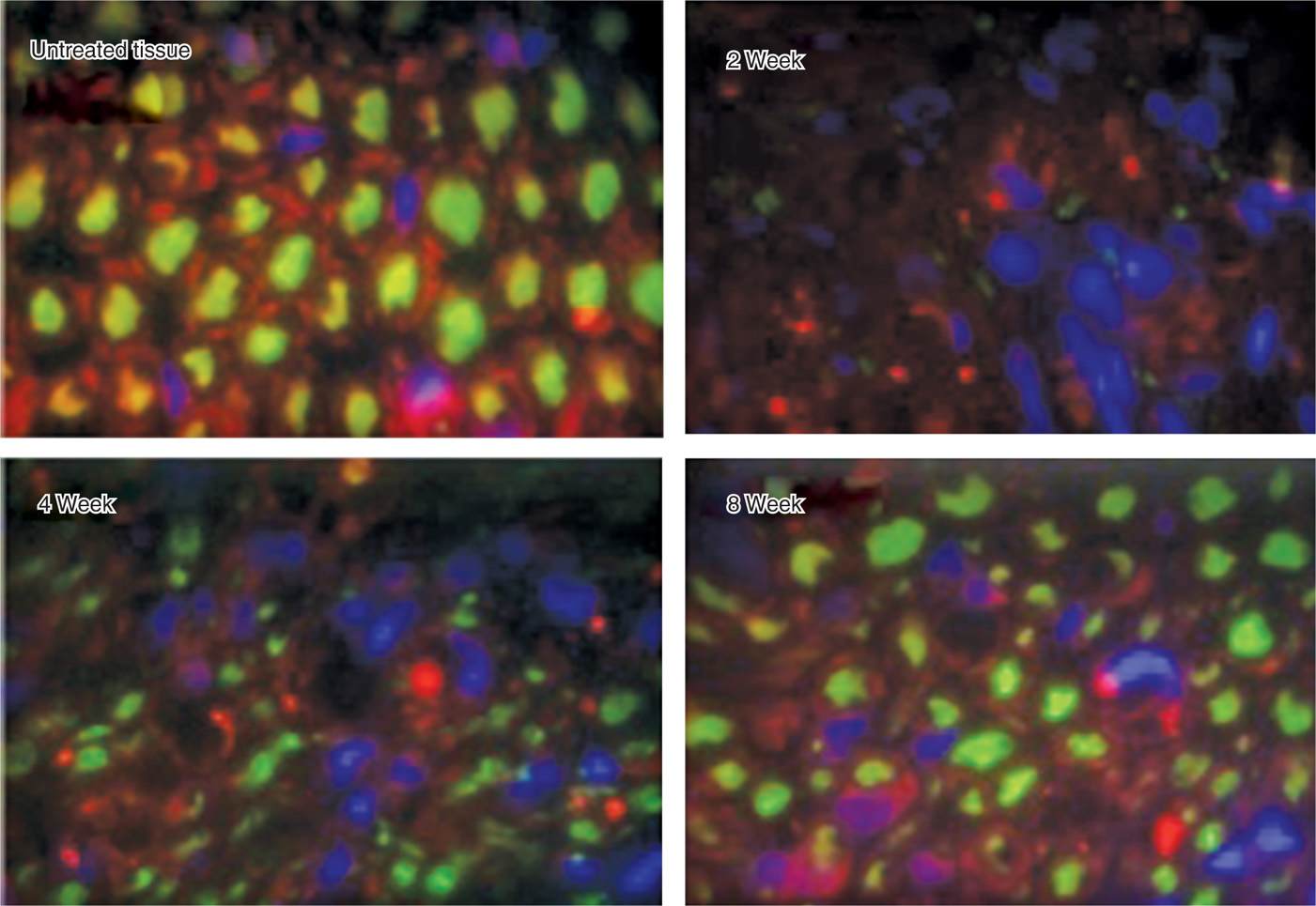
Figure 79-5. Regeneration after cryoneuroablation. (Reproduced with permission from Myoscience, Redwood City, CA.)
• Although demyelination and degeneration of the axon occurs, Sir Sydney Sunderland4 in 1968 demonstrated that when the endoneurium remains intact, neuroma formation does not occur, and the nerve is typically able to regenerate at a rate of 1 to 1.5 mm/wk.5
• The intensity and duration of analgesia is dependent on the degree of nerve damage from the ice ball.
• Autoimmune phenomena are also implicated in the long-term effects of cryoneuroablation. There is a release of sequestered proteins that may trigger an autoimmune response to the targeted lesioned tissues, which might explain the prolonged effect.
The extent of the freezing (and subsequent nerve damage) is a function of:
• The proximity of the probe to the nerve
• The size of the cryoprobe
• The size of the ice ball formed
• The completeness of the freezing (rate and duration)
• The temperature of the tissues in proximity to the probe, which is affected by local heat sinks (such as CSF/blood flow)
The nerve is expected to regrow and there is no evidence of permanent neurologic damage as a result of multiple cryoneuroablation procedures. Consequently, it is critical that the period of pain relief after the cryoneuroablation be used to regenerate as normal an environment as possible so that as the nerve regenerates the original pathology (entrapment) does not recur. Cryoneuroablation may address the issue of “wind up” (the continued stimulation of a nerve causing increased sympathetic outflow and thus more stimulation), resulting in a period of reduced sympathetic stimulation that may allow aggressive rehabilitation. When working around motor nerves, it makes sense to test for motor function during the first freeze cycle. For instance, when treating the superior gluteal nerve, asking the patient to flex the knee during the first minute of freezing would identify possible unintended motor paresis before full cooling has occurred.
INDICATIONS
Cryoneuroablation is indicated for the treatment of nerve pain (neuralgia). The application of cold to tissue creates a conduction block, similar to the effect of local anesthetics, however much longer lasting. Success of cryoanalgesia is directly related to patient selection, accurate probe placement, and the postprocedure rehabilitation process.
CONTRAINDICATIONS
Absolute contraindications to cryoneuroablation include lack of consent from patient, infection at the insertion site, any allergy or sensitivity to the anesthetic agent, and distortion of anatomical landmarks.
GENERALIZED REMARKS
The cryoneurolysis technique is solely dependent on the appropriate and reliable diagnosis. It is critical that a precise diagnosis be made prior to an attempt to freeze any nerve. These diagnostic steps include:
• Identify the clinical pattern of pain; perform a meticulous diagnostic block.
• Use small volumes of local anesthetic no greater than the volume of the freeze that would be created by the ice ball (0.5-1 mL).
• Utilize a nerve stimulator as well as fluoroscopic guidance, ultrasound localization, direct exposure, or absolute anatomic location.
Success of cryoanalgesia is directly related to:
• Patient selection
• Accurate diagnosis
• Accurate probe placement
• The postprocedure rehabilitation process
The recommended technique(s) encompasses the following: meticulous localization of the nerve, the use of the largest probe appropriate, and the use of adequate freeze and defrost cycles. It is also critical that the cryoprobe be withdrawn only after the ice ball has thawed since trying to withdraw the probe with the ice ball present could tear the attached tissues and avulse a nerve segment.
COMMON TECHNICAL STEPS
• The skin is anesthetized using very small amounts of local anesthetic with special care not to anesthetize the nerve.
• Next, the subcutaneous tissues around the nerve are infiltrated with 1 to 2 mL of 0.9% preservative-free normal saline with 1:200,000 epinephrine (5 μg epinephrine per mL normal saline).
• This provides constriction of the surrounding blood vessels and will decrease bruising from the cryo probe.
• The introducer (usually an IV catheter) is gently advanced into the tissues to the target area, and tiny doses of local anesthetic added if needed to provide analgesia (taking care to avoid anesthetizing the target nerve).
• The probe is then advanced through the introducer until it lies over the nerve.
• The sensory function of the probe is used to place the probe as close as possible to the nerve, using a technique of “successive approximations”; the sensory stimulator is turned up to 1 mV and slowly moved until the patient feels the paresthesia (a tingle or burning sensation) and then immediately turned off.
• After a few seconds, the stimulator is turned back up to 1 mV; if the patient does not feel stimulation, then it was a false paresthesia.
• If the patient feels the paresthesia before 1 mV is reached, the stimulator is turned up only to 0.5 mV and the probe moved until the patient feels the paresthesia and then turned off, and the process repeated until the stimulation is felt at 0.3 mV, each time getting closer and closer to the nerve.
• The motor function is then used to confirm the lack of motor stimulation at 2 mV.
• Two or three, 2-minute freeze cycles are usually sufficient for clinical relief. Special care must be taken to make certain that a superficial “frostbite” burn of the dermis and epidermis is not occurring.
COMMON SIDE EFFECTS AND COMPLICATIONS
• Cryoneurolysis is a procedure with certain risks and potential problems.
• Every patient should be warned about possibility of bleeding with subsequent hematoma formation.
• Allergic or systemic reactions to an anesthetic agent do happen as well as unintentional injection into artery or vein.
• Infection is always possible but it can be minimized by application of judicial aseptic techniques.
• Also, the nerve can be damaged, resulting in neuralgia and exacerbation of the pain.
• The other risks for cryoneurolysis include the risk of depigmentation, hyperpigmentation, or tissue necrosis at the cryolesion site. This is sometimes compounded by alopecia.
• A separate complication is inadequate pain relief, most likely due to inadequate localization of the nerve.
Additionally, each site has potential for specific complications that will be described below.
SUPRAORBITAL NERVE
Indications
• Commonly confused with migraines and frontal sinusitis, the pain of supraorbital neuralgia most typically manifests itself as a frontal headache, often associated with blurred vision, nausea, and photophobia.
• Vulnerable to blunt trauma, this nerve can be injured by deceleration against an automobile windshield or through blows to the face.
• This neuralgia tends to worsen with time following the trauma as the injured tissue slowly develops a cicatrix, which eventually envelops the nerve.
• Spasm of the orbicularis oculi (such as with frowning or squinting) exacerbates the entrapment. This etiology is supported by the efficacy of botulinum toxin in the forehead region in the treatment of “migraines.”
• However, the botulinum relief is only expected to last 2 to 3 months, where the cryoneuroablation relief can be for up to a year or longer.
• Less commonly, the nerve can be injured as the result of acute herpetic infection (eg, shingles), Paget disease, and neoplasm.
• Patients will often experience an increase in headache intensity and frequency with menstruation (associated with fluid retention), salt intake, stress, and bright light or vision changes (which causes squinting).
Relevant Anatomy
The supraorbital nerve is the termination of the first division of the trigeminal nerve. Irritation of the nerve occurs primarily at the supraorbital notch (Figure 79-6).
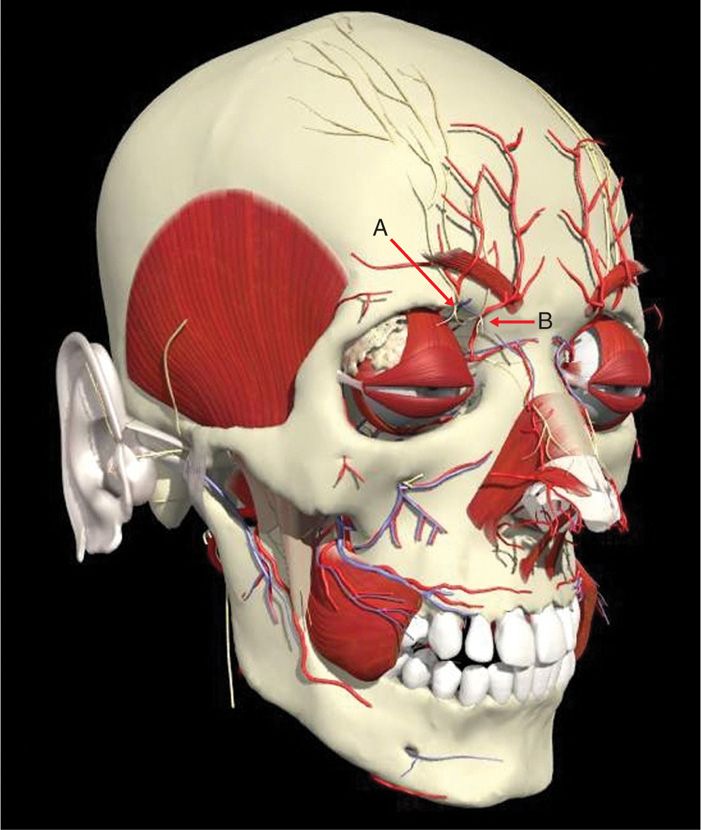
Figure 79-6. Supraorbital anatomy. A, supraorbital, nerve B, supratrochlear nerve. (Used with permission from Andrea Trescot, MD.)
• A small ligament completes the inferior border of a foramen through which the nerve passes prior to passage through the orbicularis oculi.
• Relevant anatomy includes the supraorbital notch and supratrochlear groove.
• The supratrochlear nerve is located at the medial aspect of the orbit. Considering the cosmetic aspect, a small probe should be used and the nerve should be approached from the inferior aspect.
Preoperative Considerations
Adequate preparation of the patient in respect of complications should be done, including the risk of alopecia and depigmentation or hyperpigmentation.
Fluoroscopic Views
This procedure does not usually require fluoroscopy.
Positioning of the Patient
The patient can be in place sitting or in the supine position. The supine position is favorable in case the patient has a vagal episode.
Intraoperative Technical Steps
• Cryoneuroablation of the supraorbital nerve can be accomplished via an open operative technique involving dissection under local anesthesia. The nerve can then be frozen under direct vision.
• Alternatively, the closed technique involves using the 1.4-mm probe, passed via a 14-gauge intravenous catheter introducer.
• The approach is usually inferior to the supraorbital notch.
• The probe trajectory ideally runs parallel to the nerve.
• Special care must be taken to make certain that a superficial burn of the dermis and epidermis is not occurring.
• Close observation of the skin during the entire freeze cycle is imperative (Figure 79-7).
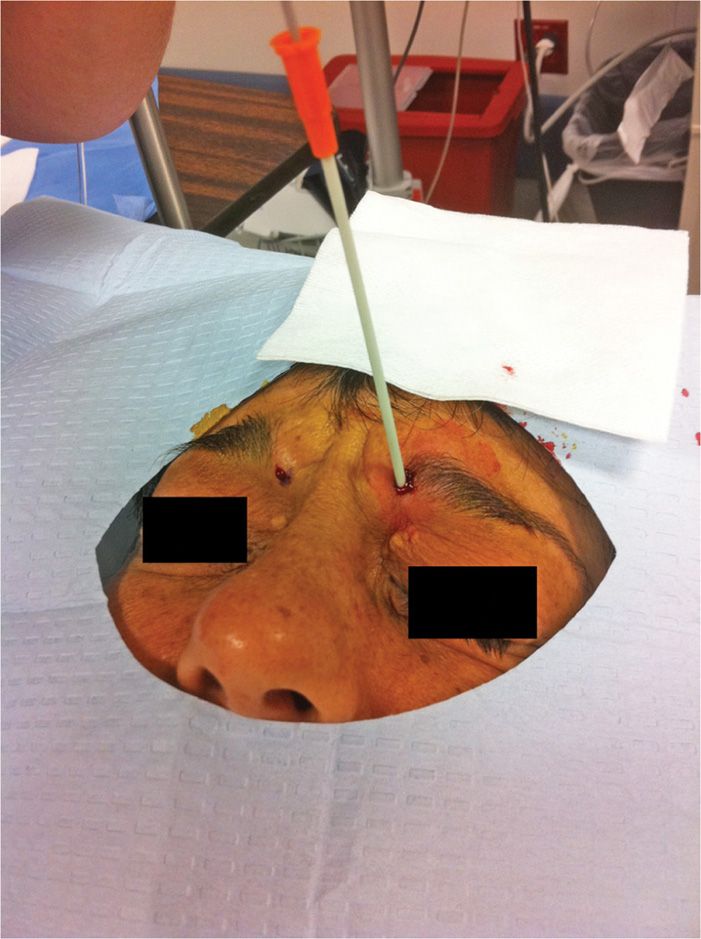
Figure 79-7. Cryoneuroablation supraorbital nerve. (Used with permission from Andrea Trescot, MD.)
Postprocedure Considerations
Changes in skin color can be expected, but generally resolve in a matter of days to months; however, the patient must be counseled appropriately.
Monitoring for Potential Complications
Swelling and bruising of the eyelid or entire eye can occur.
Clinical Pearls and Pitfalls
Entry of the catheter and probe should be below the eyebrow line. This avoids damage to the brow follicles, with subsequent alopecia.
INFRAORBITAL BLOCK
Indications
• The infraorbital nerve is the termination of the second division of the trigeminal nerve. Irritation peripheral neuropathy occurs principally at the infraorbital foramen.
• Also vulnerable to blunt trauma, this nerve is often injured by pugilistic blows. This neuralgia tends to worsen with time following the trauma as the injured tissues slowly develop cicatrix that entraps the nerve.
• This nerve can also be injured as the result of fracture of the zygoma, with entrapment of the nerve from the formation of bony callus.
• Commonly confused with maxillary sinusitis, the pain of infraorbital neuralgia typically presents as maxillary pain worsened by smiling and laughter (which puts tension on the zygomaticus musculatures).
• Because of referred pain to the teeth, patients often undergo futile dental procedures prior to their presentation.
• As with the aforementioned entrapments, these patients will often experience an increase in headache intensity and frequency with menstruation, salt intake, stress, and bright light.
Relevant Anatomy
The infraorbital nerve is the termination of the second division of the trigeminal nerve. As second division of the trigeminal nerve enters the infraorbital foramen, it is commonly called infraorbital nerve. This nerve innervates the lower eyelid, upper lip, and part of the nasal vestibule and exits the infraorbital foramen of the maxilla (Figure 79-8).
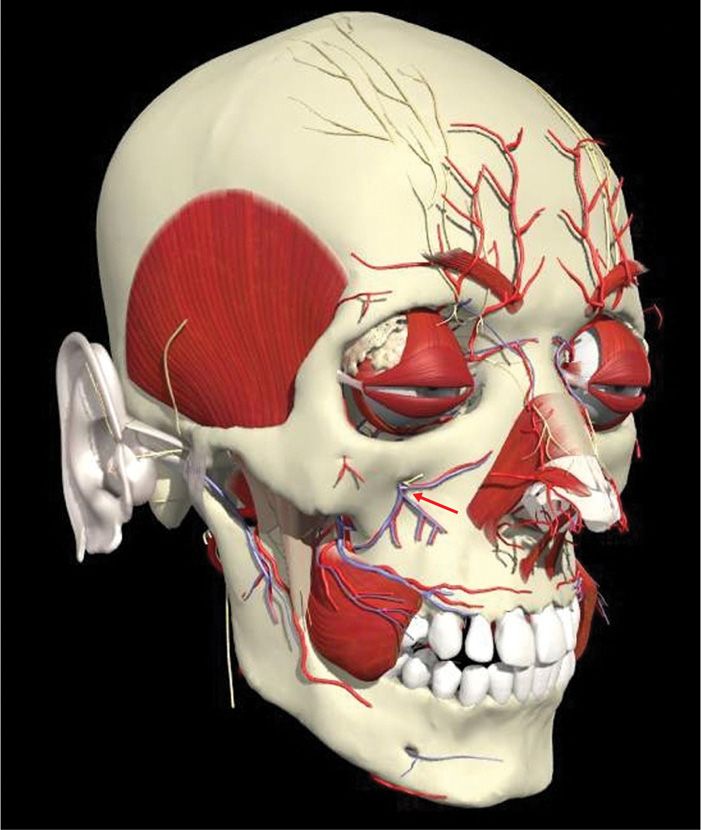
Figure 79-8. Infraorbital anatomy. (Used with permission from Andrea Trescot, MD.)
Preoperative Considerations
Fluoroscopic Views
This procedure does not usually require fluoroscopy.
Positioning of the Patient
The patient can be in place sitting or in the supine position. The supine position is favorable in case the patient has vagal episode.
Intraoperative Technical Steps
• Cryoneuroablation of the infraorbital nerve can be accomplished via an open operative technique involving dissection under local anesthesia, and the nerve can be thereby frozen under direct visualization.
• The closed technique involves using the 1.4-mm probe, passed via a 14-gauge introducer, as close as possible to the foremen.
• This can be accomplished by a direct percutaneous approach but this area is also cosmetically important, so to minimize cosmetic damage, the intraoral approach can be employed
• With the intraoral approach, a 12-gauge introducer and 2.0-mm probe are inserted intraorally through the superior buccal-labial fold (Figure 79-9).
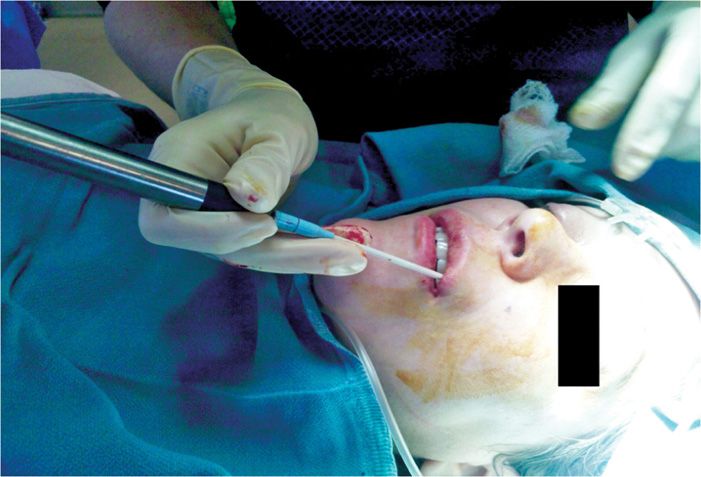
Figure 79-9. Cryoneuroablation infraorbital nerve, intraoral approach. (Used with permission from Andrea Trescot, MD.)
• If performed percutaneously, close observation of the skin during the entire freeze cycle is imperative.
Postprocedure Considerations
Changes in skin color can be expected, but generally resolve in a matter of days to months; however, the patient must be counseled appropriately.
Monitoring for Potential Complications
Swelling of the cheek up to the inferior eyelid can occur. Bleeding, infection, or hematoma formation should also be discussed as a potential risk.
OCCIPITAL BLOCK
Indications
Occipital neuralgia presents as unilateral or bilateral occipital nerve pain and headache, radiating to retro-orbital area. It is related to entrapment by the trapezius, splenius, and levator scapulae muscles and flexion/extension injuries or may be related to pathology of cervical facets.
Relevant Anatomy
The greater occipital nerve originates from dorsal ramus of C2 and C3, travels cephalad, and pierces nuchal fascia at base of skull (Figure 79-10). The fibers of C2 ganglion relay at the level of the medulla with the caudate portion of the trigeminal ganglion, and therefore refer sensations to the face and posterior eye area. It also picks up fibers from the lesser occipital nerve (from cervical plexus) and third occipital nerve (posterior ramus C3).
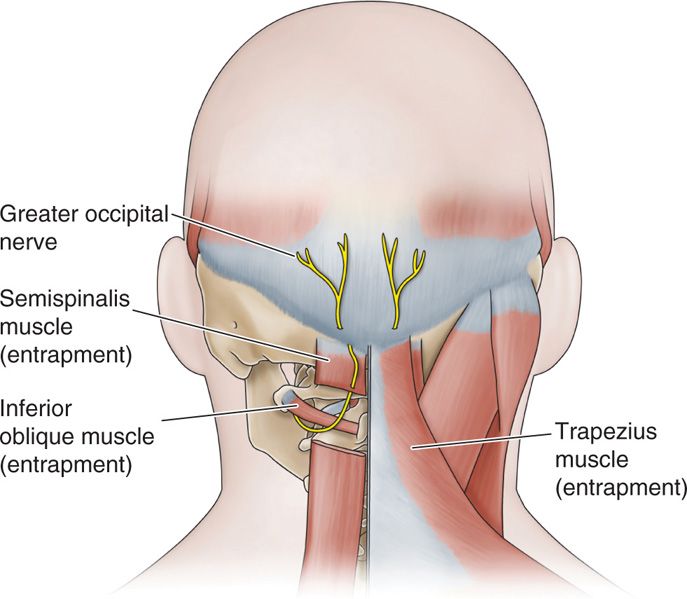
Figure 79-10. Occipital anatomy. (Reproduced with permission from Epimed, Farmers Branch, TX.)
Preoperative Considerations
Fluoroscopic Views
This procedure does not usually require fluoroscopy.
Positioning of the Patient
The patient is placed in the prone position with support under the chest on the OR table and the patient’s head flexed in a comfortable position.
Intraoperative Technical Steps
• Cryoneuroablation of the greater occipital nerve can be accomplished via an open operative technique involving dissection under local anesthesia, and the nerve can be thereby frozen under direct visualization.
• The closed technique involves using a 12-gauge introducer and 2.0-mm probe, which is inserted approximately 3 cm below the occipital protuberance and 1.5 cm lateral to the midline (Figure 79-11).
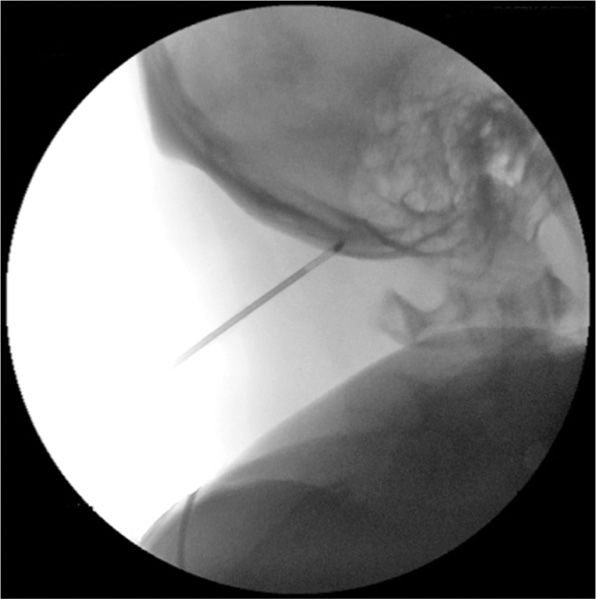
Figure 79-11. Cryoneuroablation occipital nerve (fluoroscopy). (Used with permission from Andrea Trescot, MD.)
• The probe is then advanced until it lies over the greater occipital nerve on the periosteum, which is identified by sensory stimulation.
• The probe can also be introduced under ultrasound guidance (Figure 79-12).
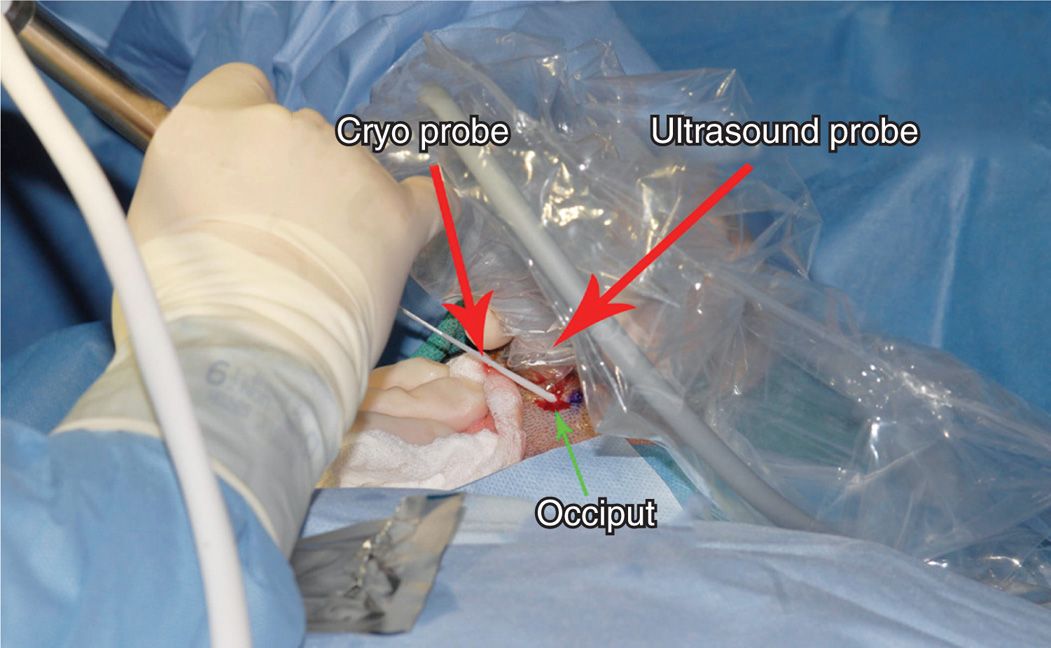
Figure 79-12. Cryoneuroablation occipital nerve (ultrasound). (Used with permission from Andrea Trescot, MD.)
Postprocedure Considerations
Although alopecia is possible, because of the position at the back of the head, it is usually not cosmetically a problem.
Monitoring for Potential Complications
Complications may include dural puncture, spinal cord trauma, subdural injection, neural trauma, injection into the intervertebral foramen and intervertebral arteries (all related to lack of knowledge of anatomy); intravascular injection into veins, vertebral arteries or occipital artery; infectious complications including epidural abscess and bacterial meningitis; and side effects related to the administration of local anesthetics and other drugs.
INTERCOSTAL BLOCK
Indications
• Of all the postoperative indications for cryoneurolysis, lesioning of the intercostal nerve intraoperatively has been the most extensively studied.
• The nerve is very easily identified at thoracotomy, and intraoperative cryoneuroablation can provide significant and long lasting postoperative analgesia.
• It is somewhat more difficult to address when there is a post-thoracotomy neuroma, persistent pain after rib fractures, or thoracic postherpetic neuralgia, but a percutaneous technique can provide excellent analgesia.
• The technique is most effective in relieving incisional pain and provides relatively little relief of visceral pleuritic pain or the pain of ligamentous or muscle pains.
• It provides no relief for chest tube pain.
• The multiple pain generators involved in post-thoracotomy pain make cryoneuroablation difficult to use as a sole treatment.
Despite these limitations, studies have shown that patients have less postoperative pain and less opioid requirement, both in the immediate postoperative period and in the weeks following the procedure. It is the experience with post-thoracotomy pain and cryoneuroablation that led to its use in other chronic chest wall pains. The complications of postoperative neuroma, costochondritis, postherpetic neuralgia, and rib fractures have all been treated with cryoneuroablation.
Relevant anatomy
The intercostal nerve lies posterior and cephalad to the inferior border of the rib (Figure 79-13).
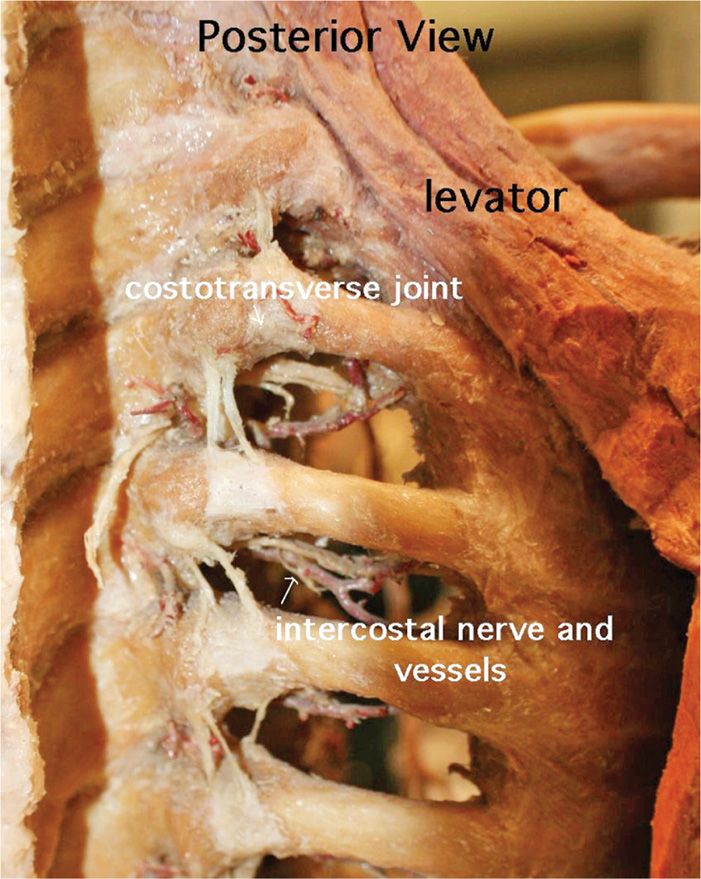
Figure 79-13. Intercostal anatomy. (Used with permission from Andrea Trescot, MD.)
Preoperative Considerations
Fluoroscopic Views
The fluoroscopic image needs to identify the ribs clearly (Figure 79-14).
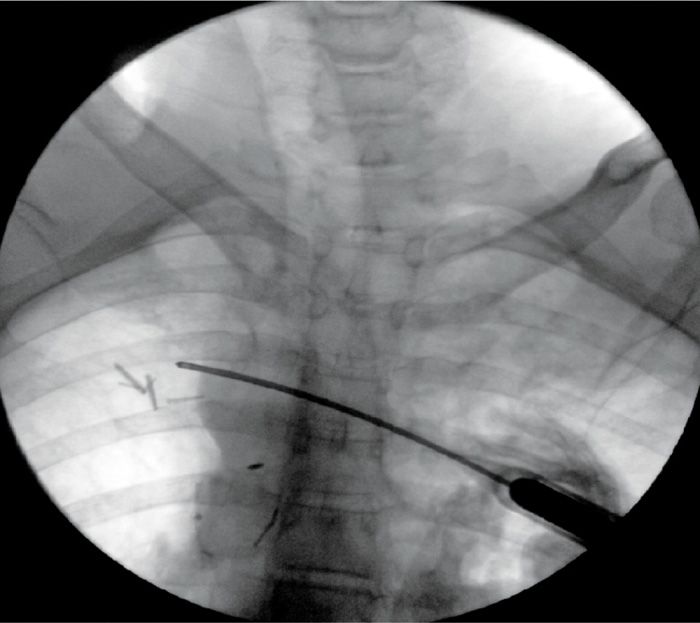
Figure 79-14. Cryoneuroablation intercostal nerve. (Used with permission from Andrea Trescot, MD.)
Positioning of the Patient
Positioning is dependent on the site of the lesion and comfort of the patient. Posterior pathology is treated from a prone position, while anterior pathology is treated in the supine position. Lateral rib pain may be best treated in a lateral position.
Intraoperative Technical Steps
• In the open technique, after the thoracotomy is complete, the cryoprobe is placed directly on the nerve at the posterior rib angle. The nerve is easily visualized beneath the parietal pleura.
• Because each rib has an innervation contribution from the rib below and the rib above, it is advisable to lesion the intercostal nerves above and below the incision line as well.
• Because the nerves are exposed, 1 or 2 short freeze cycles should be adequate.
• In the percutaneous approach, these authors recommend approaching the rib edge tangentially from medial to lateral, then pushing the tip of the probe up under the edge of the rib (Figure 79-14).
• It dramatically reduces the risk of pneumothorax, and it increases the length of contact of the probe on the nerve, increasing the effectiveness of the cryolesion.
• The 12-gauge introducer and 2.0-mm probe are favored to provide the maximal area of neurolysis.
Postprocedure Considerations
Risk of pneumothorax and bleeding is decreased with the tangential approach, with no cases of pneumothorax or bleeding after more than 50 intercostal cryoneuroablation procedures (AMT, personal correspondence).
Monitoring for Potential Complications
• Since these are primarily sensory nerves, no motor weakness would be expected from cryoneuroablation.
• Meticulous use of the motor stimulation capability should identify if the probe is too close to an unintended motor nerve.
• A postprocedure chest x-ray is critical for the evaluation for pneumothorax. Cryoanalgesia of the third and fourth intercostals can cause ipsilateral nipple anesthesia. For that reason, it has been suggested not to freeze nerves above the fifth intercostal nerve.
• Also, there has been one case report of a neuroma formation after the use of cryoneuroablation for post-thoracotomy pain and several patients who have noted sensory deficits for up to 6 months.
• Loss of tone from the external and internal oblique muscles (innervated by the lower intercostal nerves) can cause a subtle but definite subcostal bulge that resolves with the return of sensation (Figure 79-15). Patients should be made aware of that preoperatively.
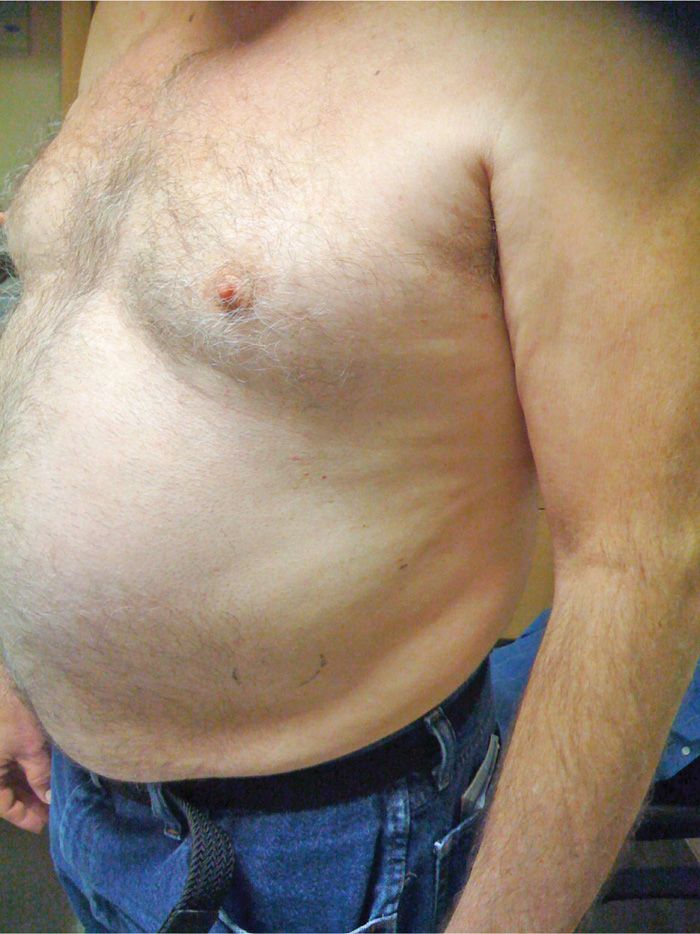
Figure 79-15. Abdominal wall bulge. (Used with permission from Andrea Trescot, MD.)
Clinical Pearls and Pitfalls
• With the percutaneous approach, the technique has to take into consideration the underlying lung and the intercostal artery, which acts as a heat sink.
• Physicians are traditionally taught to perform intercostal nerve blocks by advancing needle perpendicular to the inferior edge of rib and then “walking it off the edge of the bone,” dropping to just before the parietal pleura.
• However, the nerve is actually up under the curve of the rib. In addition, the intercostal artery acts as a huge “heat sink,” limiting the size of the ice ball and therefore the effectiveness of the cryolesion.
• By introducing the probe perpendicular to the nerve, the area of freezing is limited, which also limits the effectiveness of the cryolesion.
• In addition, the temptation is to use a smaller probe because of the concern regarding advancing a 12-gauge needle into the pleural space, causing a pneumothorax.
• The technique we recommend is somewhat different. We recommend approaching the rib edge tangentially from medial to lateral, then pushing the tip of the probe up under the edge of the rib.
• This accomplishes several things. It dramatically reduces the risk of pneumothorax, and it increases the length of contact of the probe on the nerve, increasing the effectiveness of the cryolesion.
• It is important to use the largest probe possible to overcome the arterial heat sink.
Similar treatments are available for nerves of the abdominal wall, pelvis, low back, and buttocks, as well as the upper and lower extremities; cryoneuroablation treats pain problems from the head down to the toes.
CONCLUSION
In conclusion, cryoneuroablation is an effective interventional pain management technique, providing significant analgesia in an outpatient or office setting. The technique can be utilized literally from the head down to the foot, and is limited only by the clinician’s ability to make the diagnosis of nerve pathology, and only a few of the more common procedures have been described in this chapter. The effect is routinely reversible, relatively painless, and is not associated with neuroma formation. An accurate diagnosis with specific diagnostic injections of small volumes of local anesthetic and meticulous localization of the nerve is critical for a successful outcome. Remember, “You cannot treat what you cannot diagnose.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






