KEY POINTS
Assessment of the adequacy of maternal blood flow requires an understanding that the baseline flow is substantially increased and is further augmented during labor and delivery.
The increased cardiac output of pregnancy is often diminished, especially in late pregnancy, in the supine position by uterine compression of the vena cava and abdominal aorta. Placing the patient in the left lateral decubitus position is an important management principle in shock.
The normal hyperventilation of pregnancy results in a respiratory alkalosis with a compensatory metabolic acidosis. The normal arterial blood gas values in pregnancy include a PO2>100 mm Hg, a PCO2 of 27 to 34 mm Hg, and a serum bicarbonate concentration of 18 to 21 mEq/L.
Fetal viability depends on adequate oxygen delivery. Maternal cardiac output is the critical determinant of placental blood flow and fetal oxygen delivery. Diminished placental blood flow is particularly dangerous if superimposed on maternal anemia or hypoxemia. Fetal oxygen delivery can be improved by optimizing maternal cardiac function, transfusing blood to increase oxygen carrying capacity, and providing supplemental oxygen.
In critically ill gravidas, fetal monitoring should be performed when available and in collaboration with obstetrics clinicians. Changes in fetal heart rate can be a sign of inadequate oxygen delivery. In addition to fetal heart rate monitoring, the parameters of oxygen delivery and acid-base status in the mother are generally the best measures of the adequacy of oxygen delivery to the fetus.
Hemorrhage in pregnancy can be massive and may require extraordinary fluid resuscitation, blood product replacement, and early surgical consultation.
In pregnancy, sepsis is rare but can be severe. Source control and early surgical evaluation for obstetric infections are essential. Vasoactive drugs may be indicated in refractory hypotension to preserve maternal cardiac output and fetal oxygen delivery.
Preeclampsia is a multisystem disorder of vascular dysfunction characterized by hypertension and proteinuria. Central nervous system dysfunction, coagulopathy, pulmonary edema, renal dysfunction, and liver function abnormalities may occur. Early recognition and well-timed delivery are crucial.
When severe or when associated with preeclampsia, treatment of hypertension in pregnancy may require intravenous agents: labetalol and hydralazine are preferable to nitroprusside.
Cardiopulmonary resuscitation in pregnancy includes consideration, when feasible, of emergent cesarean section in selected patients.
In evolving respiratory failure, early elective intubation and mechanical ventilation are recommended to gain airway access in a controlled setting and to avoid respiratory crisis.
The reduced functional residual capacity (FRC) and increased oxygen consumption in pregnancy increase the risk of hypoxemia during intubation or hypoventilation.
Decisions regarding labor and delivery are important management issues. Cesarean section is a more controlled mode of delivery. However, even with adequate sedation and analgesia, the physiologic stress of surgery may make vaginal delivery a better option in the nonemergent setting and when the mother is capable of labor.
The increased volume of distribution and glomerular filtration rate may affect dosing of medications in pregnancy.
Successful management of critical illness in pregnancy requires a multidisciplinary team of intensive care, pharmacy, obstetric, and neonatal consultants.
Critical illness during pregnancy requires a multidisciplinary approach that considers both the mother and the fetus. There is a paucity of definitive trials to guide therapy in critically ill pregnant patients. Measures that optimize maternal well-being are usually best for the fetus as well. Knowledge of the expected adaptations in maternal physiology is essential to distinguish between normal and pathologic findings in gravid patients. This chapter begins with an overview of the normal physiologic changes in pregnancy and the determinants of fetal oxygen delivery. The remainder of the chapter focuses on the disorders and management of critical illness in pregnancy.
PHYSIOLOGY OF PREGNANCY
In pregnancy, numerous circulatory adjustments occur that ensure adequate oxygen delivery to the fetus (Table 127-1).1 Maternal blood volume increases early in pregnancy, reaching a level approximately 40% above baseline by the third trimester.1-3 This increase is due to a 20% to 40% increase in the number of erythrocytes and a 40% to 50% increase in plasma volume; the magnitude of the increase in blood volume is even greater with multiple gestations. As the increase in plasma volume is greater than the increase in erythrocytes, a mild dilutional anemia results, with an approximate 12% decrease in hematocrit.2 Extracellular volume expansion is also associated with a decreased serum albumin concentration and colloid osmotic pressure; both indices reach a nadir at 26 weeks, although there is a further decline in colloid osmotic pressure in the immediate postpartum period.3 Extracellular volume expansion is mediated by sodium retention from increased aldosterone production, which results in mild peripheral edema in most pregnancies.2,3
Circulatory Changes in Pregnancy
| Parameter | Change | Time Course |
|---|---|---|
| Maternal blood volume | Increase 40% | Peak at 34 weeks |
| Red cell mass | Increase <20%-40% | Peak at 40 weeks |
| Hematocrit | Decrease 12% | Nadir at 30 weeks |
| Heart rate | Increase 10%-30% (15-20 bpm) | Peak at 32-36 weeks |
| Stroke volume | Increases | Increases throughout |
| Cardiac output | Increases 30%-50% | Peak at 25-32 weeks |
| Blood pressure | Decreases 10%-20% | Nadir at 28 weeks |
| Systemic vascular resistance | Decreases 20%-30% | 1st trimester |
| Pulmonary vascular resistance | Decreases 20%-30% | 1st trimester |
Coincident with increased blood volume is a 30% to 50% increase in cardiac output. This begins in the first trimester and continues throughout gestation as heart rate, and to a lesser degree stroke volume, increase.4-6 The increased heart rate reaches a maximum of 15 to 20 beats per minute above resting nonpregnant levels by weeks 32 to 36.5,7 Increased stroke volume occurs early, and is due to increased preload from augmented blood volume, and to decreased afterload from a 20% to 30% fall in systemic vascular resistance (SVR). The fall in SVR is attributed to flow through the low-resistance uteroplacental bed and to hormone-mediated vasodilation. During labor, cardiac output can increase another 10% to 15% due to increased blood return from uterine contractions, and to pain-mediated sympathetic stimulation. This effect on cardiac output may be tempered by blood loss during delivery. In healthy women, there is no substantial change in the properties of ventricular contractility over the course of pregnancy.4-6,8 Cardiac output in pregnancy can be highly dependent on body position; vena caval and aortic obstruction by the gravid uterus is maximal in the supine position but is much less pronounced in the left lateral decubitus position.2 Obstruction of the inferior vena cava results in reduced venous return, and obstruction of the abdominal aorta results in increased afterload. These effects on cardiac output are most notable in the third trimester.
Blood pressure decreases early in pregnancy, reaching a nadir between 16 and 28 weeks, and then gradually increases.9 Blood pressure returns to prepregnancy levels shortly after delivery. Hypertension in pregnancy is defined by systolic pressures >140 mm Hg or diastolic pressures >90 mm Hg. Systolic pressures ≥160 mm Hg or diastolic pressures ≥110 mm Hg define severe hypertension, and require treatment.10
The normal adaptation of the circulatory system to pregnancy results in a physiologic third heart sound in many patients. The chest radiograph reveals an enlarged cardiac silhouette due to increased circulating blood volume and cardiac filling. The pulmonary artery and right ventricular pressures are unchanged, with a hormone-mediated reduction in pulmonary vascular resistance compensating for the increased flow from augmented cardiac output. The pulmonary artery wedge pressure (Ppw) also is generally unchanged from prepartum values.3,11
Oxygen consumption is increased by 20% to 35% in normal pregnancy, and rises even further during labor (Table 127-2).12,13 Increased oxygen consumption is the result of fetal and placental utilization, as well as increased maternal requirements from increased cardiac output and work of breathing.13 Increased oxygen consumption is associated with an increase in carbon dioxide production, which reaches 34% to 50% above baseline by the third trimester. Minute ventilation increases early in pregnancy and peaks at 20% to 40% above baseline at term.3,14 The increased ventilation is above the level needed to eliminate carbon dioxide, and the arterial partial pressure of carbon dioxide (PaCO2) is reduced to 27 to 34 mm Hg throughout pregnancy. Augmented ventilation is attributed to respiratory stimulation from progesterone, and results from an increase in tidal volume while the respiratory rate is essentially unchanged.3,15 Renal compensation is associated with a decrease in serum bicarbonate to 18 to 21 mEq/L, and results in a maternal pH that is only slightly alkalemic at 7.40 to 7.45. As sodium levels are also somewhat decreased in normal pregnancy, the drop in bicarbonate is not associated with a substantial change in the anion gap.
Respiratory Changes in Pregnancy
| Parameter | Change | Time Course |
|---|---|---|
| Oxygen consumption | Increases 20%-35% | Peak at term |
| Tidal volume | Increases 30%-35% | Peak at term |
| Respiratory rate | Unchanged | |
| Minute ventilation | Increases 20%-40% | Peak at term |
| Total lung capacity | Unchanged | |
| Chest wall compliance | Decreases | |
| Lung compliance | Unchanged | |
| Functional residual capacity | Decreases 10%-25% | Peak at term |
| Forced vital capacity | Unchanged | |
| FEV1 | Unchanged | |
| Diffusing capacity | Unchanged | |
| Expiratory reserve volume | Decreased | 2nd half of pregnancy |
| Residual volume | Decreased | 2nd half of pregnancy |
Due to augmented minute ventilation, the maternal arterial partial pressure of oxygen (PaO2) is increased throughout pregnancy to greater than 100 mm Hg. As it does not alter the degree of hemoglobin saturation, this increase in PaO2 does not significantly increase oxygen delivery. An increased alveolar-to-arterial oxygen gradient [(A−a)O2] with mild hypoxemia may occur in the supine position.16 Whenever possible, arterial blood gas samples should be obtained in the seated position to avoid the confounding effect of positional hypoxemia.
Lung compliance is unchanged in pregnancy. However, elevation of the diaphragm from the enlarging uterus leads to decreased chest wall compliance. This results in a progressive decline in the functional residual capacity (FRC), which reaches a 10% to 25% reduction by term.3,15 Expiratory reserve volume and residual volume are decreased during the second half of pregnancy.15 Total lung capacity, however, decreases minimally as respiratory muscle function is unimpaired, and widening of the thoracic cage increases inspiratory capacity.12,15 Vital capacity also remains unchanged during pregnancy. Diffusing capacity is unchanged or slightly increased early in pregnancy and then decreases to normal or slightly below normal after the first trimester.17 Airway closure may occur near or above FRC in some women late in pregnancy.17,18 The decrease in FRC combined with an increase in oxygen consumption make the pregnant woman and the fetus more vulnerable to hypoxia in the event of hypoventilation or apnea. This is an important consideration during endotracheal intubation. Despite increases in several hormones known to affect smooth muscle, airway function does not appear to be altered in pregnancy. Accordingly, the forced expiratory volume in 1 second (FEV1), the ratio of FEV1 to forced vital capacity, and the airways resistance are unchanged. The fact that flow-volume loops are also unaffected by pregnancy is further evidence of normal airway function.19
Renal blood flow increases throughout the first and second trimesters to reach 60% to 80% above prepregnancy levels.3 The glomerular filtration rate rises early in pregnancy to 50% above baseline, and remains increased throughout pregnancy.20 Therefore, the usual serum creatinine is 0.5 to 0.7 mg/dL, and creatinine levels that would be normal in a nonpregnant patient can indicate renal dysfunction in a pregnant patient.
Serum aminotransferases and bilirubin are unchanged in normal pregnancy. Alkaline phosphatase, produced by the placenta, increases throughout pregnancy, peaking at two to four times normal at term. The concentration of serum albumin is mildly decreased throughout pregnancy as a result of hemodilution. Symptomatic gastroesophageal reflux is common during pregnancy, although basal gastric acid secretion and pH remain unchanged.21 The lower esophageal sphincter tone decreases during the first trimester and remains low until near term, perhaps as a result of increased progesterone. The gravid uterus displaces the stomach, further reducing the effectiveness of the gastroesophageal sphincter. In addition to sphincter incompetence, labor and narcotic analgesics contribute to an increased risk of aspiration from delayed gastric emptying. When being evaluated for intubation, pregnant patients are considered to have a full stomach, regardless of the timing of the last meal.
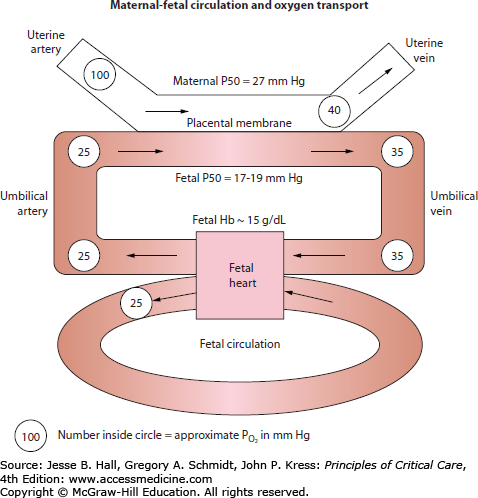
Fetal oxygen delivery is determined by uterine artery blood flow, maternal PaO2, and the hemoglobin concentration (Fig. 127-1).3 Numerous factors affect uterine artery blood flow. The uterine vasculature is maximally dilated under normal conditions and, therefore, is unable to adapt to stress by increasing flow through local vascular adjustment.22 However, fetal oxygen delivery can be decreased by uterine artery vasoconstriction. Exogenous or endogenous sympathetic stimulation, maternal hypotension, and maternal alkalemia elicit uterine artery vasoconstriction.3
In the placenta, a concurrent exchange mechanism, driven by the difference in oxygen tension between maternal and fetal blood, results in transfer of oxygen from the maternal to fetal circulation.3 Equilibration is incomplete, and umbilical venous blood going to the fetus has a lower oxygen tension than blood in the uterine vein. Oxygenated umbilical venous blood has a PO2 of 30 to 40 mm Hg, and combines with deoxygenated blood in the fetal inferior vena cava to result in a fetal arterial PO2 of 20 to 25 mm Hg.3 Despite a low PO2, compensatory mechanisms maintain good oxygen delivery, and fetal oxygen content is relatively high. Fetal hemoglobin has a higher affinity for oxygen than maternal hemoglobin, and is 80% to 90% saturated at a PO2 of 30 to 35 mm Hg.3 In addition, the fetus has a high hemoglobin concentration (15 g/dL) and a high cardiac output, with both the left and right ventricles delivering blood to the systemic circulation as a result of intrapulmonary shunting. Protective mechanisms in the fetus enable tolerance of hypoxemia that would be catastrophic by adult criteria: generally, oxygen supply only becomes inadequate when fetal oxygen content is reduced by more than 75%, and irreversible fetal brain damage begins only after 10 minutes of anoxia.3 Protective mechanisms include a redistribution of blood flow to vital organs, decreased oxygen consumption, and the ability of anaerobic metabolism to sustain certain tissue beds.
CIRCULATORY DISORDERS OF PREGNANCY
Hypoperfusion and preeclampsia are the principal circulatory disorders in pregnancy. Common causes of hypoperfusion include hemorrhage, trauma, cardiac dysfunction, and sepsis. Each is discussed below. The pathophysiology and treatment of preeclampsia is also reviewed. Together these circulatory disorders account for the majority of ICU admissions and maternal deaths related to pregnancy (Tables 127-3 and 127-4).23-25 We conclude this section by reviewing cardiopulmonary resuscitation in pregnant patients.
Indications for ICU Care in Obstetric Patients
| Diagnosis | Percent of Admissions |
|---|---|
| Obstetric | |
| Preeclampsia | 20% |
| Eclampsia | 15% |
| HELLP syndrome | 2% |
| Major hemorrhage | 16% |
| Sepsis of pelvic origin | 16% |
| Septic abortion | 12% |
| Nonobstetric | |
| Sepsis | 10% |
| Pneumonia | 6% |
| Urosepsis | 2% |
| Other | 2% |
| Respiratory failure | 4% |
| Intracranial hemorrhage | 3% |
| Other | 4% |
Causes of Maternal Mortality
| Cause of Death | Percent of Deaths |
|---|---|
| Hemorrhage | 13% |
| Cardiomyopathy | 12% |
| Hypertensive disordersa | 12% |
| Other cardiovascular conditions | 12% |
| Infection | 11% |
| Thromboembolism | 10% |
| Stroke | 6% |
| Amniotic fluid embolism | 8% |
| Anesthesia | 1% |
| Other | 13% |
| Unknown | 2% |
The initial approach to the hypoperfused gravida is to distinguish between low-flow states caused by inadequate circulating volume or reduced cardiac output, and high-flow states such as sepsis. While making this distinction, it is important to take into account the normal physiologic alterations of pregnancy (Table 127-1). Most often, the state of perfusion can be determined by bedside assessment (Fig. 127-2). Occasionally, the adequacy of the intravascular volume remains unclear despite a careful physical examination and review of laboratory data. Bedside echocardiography has emerged as a first-line procedure which is often a safe and reliable alternative to invasive monitoring for the evaluation of hypotension or refractory heart failure. In a heterogeneous group of critically ill obstetric patients, left and right ventricular function by echocardiography correlated with pulmonary artery catheter results.26 While right heart catheterization may be considered in special circumstances, a survival benefit from this invasive procedure has not been confirmed for critically ill patients, and it is not routinely recommended for obstetric patients.27 When performed on gravidas, insertion of a pulmonary artery catheter is via the subclavian or internal jugular approach. Uterine obstruction of the vena cava and delivery considerations are relative contraindications to femoral vein catheterization.
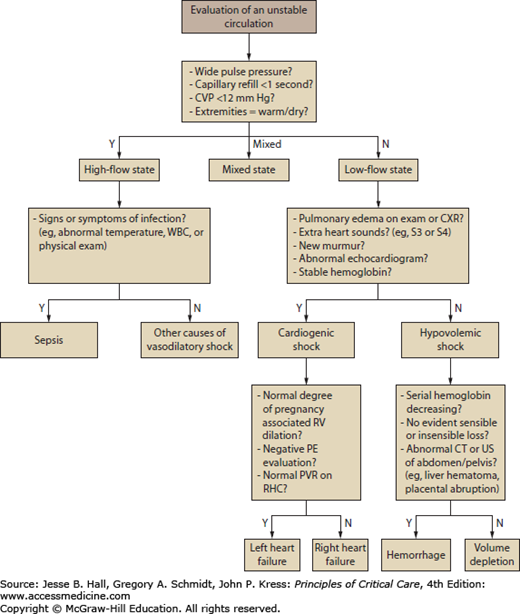
FIGURE 127-2
An approach to the evaluation of shock in pregnancy is presented. Differentiating between a high-flow and low-flow state is important in the initial assessment of the patient with an unstable circulation. Shock in a high-flow state is most often due to sepsis. Shock in a low-flow state is attributable to impaired cardiac output or a depleted circulating volume. CVP, central venous pressure; CXR, chest x-ray; PE, pulmonary embolism; PVR, peripheral vascular resistance; RHC, right heart catheterization; RV, right ventricle; WBC, white blood cells.
For pregnant patients, life-threatening hemorrhage is a leading cause of ICU admissions and death.23,24,28,29 Blood loss during labor normally does not exceed 500 to 1000 mL.30 In pathologic obstetric hemorrhage, blood loss can be massive and swift, as it occurs at sites of high blood flow. Early obstetric hemorrhage may be difficult to recognize, as it does not always result in external blood loss. In addition, fluid shifts in the immediate postpartum period can make identification of a dropping blood count difficult, and the hemoglobin concentration may be normal or unchanged initially.28 Therefore, any concerning change in maternal heart rate or blood pressure should prompt an evaluation for hemorrhage. Table 127-5 lists the common causes of hemorrhage associated with pregnancy. Antepartum hemorrhage is most often due to placental abruption, placenta previa, or uterine rupture. Postpartum hemorrhage is more common than antepartum hemorrhage, and is most often due to uterine atony or obstetric trauma; uterine inversion and disseminated intravascular coagulation (DIC) are less common causes of postpartum hemorrhage.1,3 These conditions are reviewed below. Other less common but important causes of hemorrhage in pregnancy include hemorrhage associated with ruptured ectopic pregnancy or complicated abortion.
Placental abruption is the premature separation of a normally implanted placenta, and may result in life-threatening hemorrhage and/or fetal demise. Patients often present with painful bleeding, which may be misdiagnosed as premature labor, and increased uterine activity may be detected. Ultrasound is diagnostic.31 Risk factors for placental abruption include chronic or pregnancy-related hypertension, high parity, cigarette smoking, cocaine use, and previous abruption.32-35 Abruption may be complicated by maternal renal failure or DIC.3,34 Bleeding concealed within the uterus is particularly high risk for fetal death as several liters of blood loss may go unrecognized.31,34
Placenta previa is the abnormal inferior attachment of the placenta in the uterus, which is at risk of tearing during cervical dilation. This is now a rare cause of massive hemorrhage as ultrasound during pregnancy leads to early identification and expectant management. Placenta previa is more common in multiparas with prior cesarean delivery and in cigarette smokers.1,32,36 Vaginal examination that disrupts the placenta over the cervical os, and trophoblastic tissue that invades the myometrium (placenta previa et accreta) increase the risk for massive hemorrhage at delivery.33 The associated fetal mortality is low, but increases if maternal shock occurs.
Uterine rupture can result in massive hemorrhage. Uterine abnormalities, including scarring from prior cesarean section, increase the risk of rupture. Other risk factors include protracted labor, device-assisted vaginal delivery, and use of uterotonic medications.36 Uterine rupture most often occurs during labor and delivery, although occurrence before the onset of labor has been reported.37 In overt rupture, peritoneal signs and hemodynamic instability are often observed. However, rupture at scar sites may be incomplete, and associated with painless hemorrhage and a more subtle clinical presentation.31 As the associated physical examination may be notable for only subtle changes, unexplained abnormalities in fetal heart rate or uterine contractility patterns should prompt an evaluation for rupture.31
Postpartum hemorrhage is defined by loss of over 500 mL of blood within the first 24 hours after vaginal delivery, or over 1000 mL after cesarean section. Uterine atony is the most common cause of postpartum hemorrhage. Uterine atony is associated with uterine overdistension, placental abruption, retained intrauterine contents, quick labor and delivery, prolonged labor, oxytocin use, cesarean section, and chorioamnionitis. Following delivery, coordinated myometrial contractions are needed to compresses uterine vessels and stanch hemorrhage from placental separation.28 Large clots and retained placental tissue interfere with normal myometrial contractions. A stunned or exhausted uterus from a precipitous or prolonged labor, respectively, may also experience ineffective myometrial contractions after placental delivery.38 Ultrasound is diagnostic for retained tissue or dysfunctional postpartum contractions.
Other common causes of postpartum hemorrhage include cervical or vaginal lacerations, and bleeding from uterine incisions after cesarean section. Blood loss in these cases can accumulate in the floor of the pelvis or within the uterine wall, and the lack of evident bleeding does not rule out severe hemorrhage.31 Uterine inversion may also result in hemorrhage. However, the associated hypotension is often vasovagal and out of proportion to blood loss.38,39 Uterine inversion is recognized by the presence of a blue-gray vaginal protrusion.
DIC is a syndrome of systemic coagulation activation and vascular fibrin deposition, which results in a consumptive coagulopathy. In spite of an increased plasma volume and resultant hemodilution, in normal pregnancy the levels of fibrinogen and many clotting factors are elevated. These hypercoagulable conditions notwithstanding, hemorrhage from a massive consumptive coagulopathy is the most common serious manifestation of pregnancy-associated DIC.40 Mediated by the release of procoagulant material into maternal circulation, risk factors for DIC include placental abruption, amniotic fluid embolism, fetal death, saline solution abortion, sepsis, and preeclampsia with the hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.40 DIC may occur before or after delivery, and the onset is often abrupt. The course may be fulminant and associated with high rates of maternal and fetal mortality. If the peripheral blood smear, platelet count, prothrombin time (PT), partial thromboplastin time (PTT), or fibrinogen level suggest DIC, plasma levels of fibrin degradation products and specific factors, including factor VIII, should be measured. As circulating fibrinogen levels are increased in pregnancy, especially in later stages, a “normal” level can be concerning.40
Management: Patients at risk of bleeding should be identified early for blood typing and to establish intravenous access. The initial management of hemorrhage includes maintenance of several large-bore (16-gauge or larger) intravenous catheters, immediate volume replacement with crystalloid, and administration of supplemental oxygen. For brisk bleeding, a fall in hemoglobin, or evidence of shock, packed red blood cells (PRBCs) should be given immediately. In massive obstetric hemorrhage, the initial resuscitation may require unmatched, type-specific blood until cross-matching can be accomplished; in critically urgent situations, group O RhD-negative blood can be used.41 Massive blood loss results in a dilutional coagulopathy and thrombocytopenia. Transfusion of fresh frozen plasma (FFP) is often indicated, although the optimal ratio of PRBCs to FFP is not known. A ratio of 6:1 is reasonable for most cases of obstetric hemorrhage; in reference to outcomes and practices in military trauma, some advocate a lower ratio in massive hemorrhage.42,43 During active hemorrhage, low fibrinogen and platelet levels <50,000 are indications for cryoprecipitate and platelet transfusions, respectively.42 Recombinant activated factor VIIa has been used with success in case reports of severe postpartum hemorrhage, and can be considered in refractory cases.42 The antifibrinolytic agent tranexamic acid has been used in the prevention of postpartum hemorrhage, and a large-scale study is underway to evaluate its use in treating postpartum hemorrhage.42 Finally, an evaluation for DIC should be performed in cases of severe, refractory, or unexplained hemorrhage.
When hemorrhage results in shock that is not quickly reversible or is accompanied by respiratory dysfunction, intubation and mechanical ventilation are indicated as hypoxemia superimposed on a low-flow state is injurious to the fetus and mother. If delivery has not yet occurred, the patient should be placed in the left lateral decubitus position to attenuate vena caval obstruction, which exacerbates an already reduced venous return from massive hemorrhage. Fetal monitoring is recommended as fetal distress in the setting of obstetric hemorrhage indicates hemodynamic compromise.3
An obstetric evaluation should be sought as soon as hemorrhage is suspected or recognized. A thorough pelvic examination is necessary to identify potential bleeding sources; general anesthesia may be necessary to facilitate this.31 Uterine atony is treated with bimanual uterine massage, bladder drainage, uterotonics, and removal of any retained placental products.36,39 A full bladder may impede uterine contractions, and bladder catheterization is indicated if a patient is unable to void. Intravenous oxytocin is a first line uterotonic, although its use indicates monitoring for hyponatremia. Ergot preparations are alternative first line agents; since these can raise blood pressure and have been associated with cerebral hemorrhage, they are contraindicated in hypertensive states.39 Prostaglandins, such as misoprostol, are other alternative agents.36 While generally well tolerated, prostaglandins may cause hypotension, bronchoconstriction, or intrapulmonary shunt, and should be avoided in patients with underlying cardiac or pulmonary disease.28,30 Retained placental products require curettage. For refractory bleeding due to uterine atony or pelvic trauma, selective arterial embolization, uterine suturing, uterine packing or balloon tamponade are interventional options that should be considered early.28 If these measures fail and bleeding is life threatening, hysterectomy may be necessary.44 Urgent hysterectomy in the setting of uncontrollable postpartum hemorrhage is high risk but can be lifesaving.
Trauma is a leading nonobstetric cause of maternal mortality. Motor vehicle accidents, falls, and assaults account for the vast majority of trauma cases that result in hospital admission.45 Gun-shot wounds, suicide attempts, and burns are less common. Maternal deaths are most often due to head injury or hemorrhagic shock.46 Fetal deaths are most often the result of injuries related to motor vehicle accidents (82%), gunshot wounds (6%), or falls (3%).47 The risk of maternal and fetal death is greatest for unrestrained passengers in a motor vehicle accident.48 As the gravid uterus grows, it is increasingly susceptible to deceleration injury, and increasingly likely to be directly damaged by blunt or penetrating trauma. As borderline tachycardia and supine hypotension in late pregnancy from uterine obstruction of the vena cava do not necessarily indicate blood loss, the identification of a decreased circulating volume in the gravid trauma patient can be difficult.46 When possible, left lateral displacement of the uterus will be helpful in the evaluation. Pregnancy may also mask findings of peritoneal irritation, and a high index of suspicion is warranted for mild peritoneal signs.
Preterm labor is the most common complication of trauma, occurring in 6% of pregnant trauma patients.49 Premature rupture of membranes and direct fetal injury are less common complications. Hemorrhage is the most common serious complication of trauma, accounting for most cases of maternal and fetal demise. The cephalad displacement of abdominal contents in pregnancy increases the risk of visceral injury, including splenic rupture, from penetrating trauma of the upper abdomen. Beyond 12 weeks of gestation, the urinary bladder is also a target for injury as it is displaced into the abdominal cavity. Placental abruption is the leading cause of trauma-related obstetric hemorrhage. Rapid deceleration injury can cause placental abruption as a result of deformation of the elastic uterus around or away from the less elastic placenta. Abruption may manifest in vaginal bleeding, abdominal cramps, uterine tenderness, amniotic fluid leakage, and maternal hypotension, but these findings are not as reliable as widely believed and the clinical signs may be subtle; cardiotocographic fetal monitoring is recommended as the most reliable way to detect abruption.49 Uterine rupture is a less common complication of trauma, but can be catastrophic. A direct, forceful blow to the abdomen is an important risk factor for uterine rupture.46 Finally, traumatic injury can result in fetomaternal hemorrhage, whereby blood loss from the fetal to maternal circulation occurs. Rh sensitization of Rh-negative patients, neonatal anemia, fetal cardiac arrhythmias, and fetal exsanguination are potential complications of fetomaternal hemorrhage.50
A high Injury Severity Score, a low Glasgow Coma Score, acidemia, hypotension, and fetal bradycardia are all associated with an increased risk of fetal death.49 However, a low injury severity score does not rule out fetal compromise, and performing a fetal assessment is recommended for most pregnant patients with trauma.46 There is some literature to suggest that antenatal trauma may be a cause of cerebral palsy; this observation awaits confirmation and further investigation.51
Management: The initial management of pregnant trauma patients is similar to that of other trauma patients, with a few important considerations. If airway management is required, it should be performed by an experienced individual. Resuscitation should be directed at maintaining the expanded circulating volume of pregnancy. Left uterine displacement should be performed whenever indicated and permitted by the clinical situation. If there is no overt vaginal bleeding, a pelvic examination should be performed to evaluate for tenderness, or for the presence of blood, urine, or amniotic fluid; nitrazine paper can identify amniotic fluid and confirm rupture of amniotic membranes. The diagnosis of pelvic or abdominal injury can usually be made by imaging, where computed tomography is generally more sensitive than ultrasonography.52 The approach to fetal assessment is guided by gestational age. If the estimated gestational age is less than 20 to 23 weeks, fetal heart tones should be interrogated; ultrasound is often the most effective way to accomplish this. When the estimated gestational age is greater than 23 weeks, cardiotocographic monitoring, which includes measurement of uterine activity and Doppler assessment of fetal cardiac activity, should be performed for at least 4 hours. Cardiotocographic monitoring can identify uterine contractions, placental abruption, or signs of fetal distress. Fetomaternal hemorrhage is identified by the Kleihauer-Betke test, a test for fetal hemoglobin in the maternal circulation. Maternal Rh sensitization can be prevented by administration of RhO (D) immune globulin.
If maternal death occurs despite aggressive resuscitation and if the fetus is alive and undelivered, immediate consideration should be given to postmortem cesarean section. In a review of over 150 cases, the outcomes of postmortem cesarean section were significantly related to gestational age, and were inversely related to the length of time between maternal death and delivery.53
Cardiac dysfunction in pregnancy may be due to pre-identified or de novo conditions. In addition, prior subclinical heart disease may manifest for the first time as a result of the increased cardiovascular demands of pregnancy. Cardiac dysfunction is associated with increased maternal and fetal morbidity and mortality, and is an increasing cause of critical illness in pregnancy.
More patients with congenital heart disease are surviving to reproductive age.54 Complication rates of congenital heart disease in pregnancy are substantial. In the mother, pulmonary edema and arrhythmias are common, whereas preterm delivery, small for gestational age, and respiratory distress are the most common complications in the neonate. Risk factors for adverse outcomes in those with congenital heart disease include prior cardiac events, poor baseline functional class (New York Heart Association class III or IV), cyanosis, significant aortic or mitral stenosis, or left ventricular systolic dysfunction. Mortality rates in recent reports are generally low, which likely reflect discouragement of pregnancy for those with advanced cardiac dysfunction, and improved management of those who do become pregnant. However, patients with Eisenmenger syndrome, cyanosis, or pulmonary hypertension continue to have a high pregnancy-associated mortality.55,56
Pregnancy can exacerbate or unmask underlying pulmonary hypertension; more rarely pulmonary hypertension begins in pregnancy.57 In the setting of pulmonary hypertension, the normal pregnancy-associated decrease in pulmonary vascular resistance does not occur, so that increased blood flow results in pulmonary pressures that can be even higher than baseline. Combined with increased myocardial demand from augmented cardiac output, and increased preload from an expanded circulating volume, higher pulmonary pressures can result in florid right heart failure. Labor and delivery are marked by a further increase in myocardial oxygen consumption, in addition to large fluid shifts from blood loss and the “auto-transfusions” of uterine contractions. Accordingly, the immediate postpartum period is a particularly high-risk time for decompensation.58
De novo cardiac conditions of pregnancy include peripartum cardiomyopathy, myocardial ischemia, coronary or aortic dissection, and endocarditis. Peripartum cardiomyopathy develops in up to 1 in 1300 deliveries. Postulated risk factors include African American ancestry, advanced maternal age, multiple gestations, preeclampsia, and gestational hypertension.59 While peripartum cardiomyopathy can occur in the last month of pregnancy and up to 5 months after deliver, the immediate postpartum period is the most common time for presentation.60,61 The presentation can be fulminant, with some patients requiring cardiac transplantation, although most often the clinical course is marked by the gradual recovery of ventricular function. An implantable defibrillator may be indicated during the recovery period.62 Myocardial infarction is uncommon during pregnancy, although the incidence may be increasing coincident with a higher burden of cardiovascular comorbidities in the general population. Maternal mortality is high in patients delivering within two weeks of a myocardial infarction.63 Aortic or coronary artery dissection can occur during pregnancy, and may be related to hormonal factors and increased shear stress from augmented cardiac output.63,64 Risk factors for dissection include older age, multiparity, trauma, hypertension, connective tissue disease, hypothyroidism, coarctation of the aorta, or a bicuspid aortic valve. Aortic dissection presents most commonly during the third trimester, often as a tearing interscapular pain. Pulse asymmetry or signs of aortic insufficiency may be noted on examination. Coronary artery dissection typically presents with chest pain and ischemic electrocardiogram (ECG) changes.64 In pregnant patients with no atherosclerosis risk factors who present with acute chest pain and signs of ischemia, coronary dissection should be strongly considered and thrombolytics should be avoided until angiography has been performed. Finally, bacterial endocarditis is rare in pregnancy. It occurs most often, although not exclusively, in patients with preexisting cardiac abnormalities.65 Intravenous drug use is a strong risk factor. Surgical repair should be considered without delay for fastidious organisms or severe valvular regurgitation.61
Management: For patients presenting with signs of cardiac disease, a chest radiograph and ECG are imperative. Echocardiography can detect valvular abnormalities or myocardial dysfunction. Transesophageal echocardiography and magnetic resonance imaging are the most sensitive and specific tests for aortic dissection, although computed tomography is often more readily available.63 For suspected pulmonary hypertension, an echocardiogram is often the initial test of choice, although it can both under and overestimate pulmonary pressures. Right heart catheterization is indicated for further evaluation if clinical suspicion is high, or to confirm elevated pressures noted on echocardiogram.57 In patients presenting with ischemia, a troponin level should be checked, and cardiology consultation considered early. A high B-type natriuretic peptide (BNP) level suggests cardiac strain or dysfunction.
Volume status should be optimized. For cardiogenic pulmonary edema or right heart failure, diuretics can be given as clinically indicated; the starting dose is often low in consideration of the increased glomerular filtration rate and to avoid abrupt changes in the circulating volume. Refractory cardiogenic shock is often an indication for dobutamine. For cases of severe pulmonary hypertension, intravenous prostacyclin has been given without fetal harm.66 For suspected acute myocardial infarction, diagnostic and therapeutic angiography is strongly preferred to thrombolytic therapy, which is a risk for hemorrhage and is contraindicated in ischemia due to dissection; abdominal shields during angiography reduce the risk of fetal radiation.67 Extended use of low dose aspirin has been safe in pregnancy. However, doses over 150 mg/d are cautionable, and clear recommendations for aspirin dosing in acute ischemia are lacking. Limited data on clopidogrel and glycoprotein IIb/IIIa inhibitors in pregnancy often preclude their use in pregnant patients. While nitrates and most β-blockers are considered reasonably safe in pregnancy, statin medications are contraindicated.67,68
Labor and delivery are high risk for women with cardiovascular disease. The optimal delivery method in most cases is assisted vaginal delivery in the left lateral decubitus position.60 Epidural anesthesia mitigates the tachycardic response to pain.2 Indications for cesarean section include obstetric complications, fetal distress, or inability to tolerate labor and delivery.2 In addition, general anesthesia and surgical delivery may be preferred for patients with hypertrophic cardiomyopathy, aortic stenosis, or pulmonary hypertension, conditions which place the patient at particular risk of decompensation during the increased cardiac demand and large fluid shifts of labor and delivery.2 If possible, labor and delivery should be avoided for at least two weeks following an acute myocardial infarction; aggressive antiplatelet therapy can be a contraindication for vaginal delivery.
Sepsis remains an important cause of hypoperfusion and critical illness in pregnancy, and the associated maternal mortality rate of up to 13% is high for an otherwise healthy population.23,69 Sepsis during pregnancy can be complicated by shock, acute respiratory distress syndrome (ARDS), multi-organ system failure, cardiac dysfunction, premature delivery, fetal demise, and neurological abnormalities in the infant.69,70 The hemodynamics of sepsis are similar in pregnant and nonpregnant patients. However, as normal pregnancy is associated with a decrease in vascular resistance and an increase in heart rate, determining if hypotension or pathologic tachycardia is present can be difficult. Rapid or major changes in hemodynamics are significant findings that can be concerning for sepsis. While a mild elevation in the white blood cell count is a normal finding in late pregnancy, a significant elevation or a left shift on the differential should raise concern for infection.71 Infections that cause sepsis may be obstetric or nonobstetric in nature, as reviewed below and in Table 127-6.
Causes of Sepsis in Obstetric Patients
| Obstetric | Nonobstetric | Procedure Related |
|---|---|---|
|
|
|
Obstetric infections include endometritis, septic abortion, chorioamnionitis, intra-abdominal or pelvic abscesses, or surgical site soft tissue infections, including necrotizing fasciitis.72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree