Critical Care of Hematopoietic Cell Transplant Recipients
Marco Mielcarek
I. GENERAL PRINCIPLES
A. Definition.
1. Hematopoietic cell transplantation (HCT) is a potentially curative treatment for malignant and nonmalignant diseases including leukemia, lymphoma, multiple myeloma, aplastic anemia, hemoglobinopathies, and congenital immune deficiencies.
2. Chemoradiation is used to eradicate the underlying disease and is followed by intravenous infusion of the stem cell graft.
3. Among other cells, the graft contains the stem cells that reconstitute the ablated hematopoietic system.
4. Immunosuppressive therapy is required after allogeneic HCT to prevent graft-versus-host-disease (GVHD) and graft rejection.
B. Classification: the type of HCT used is a complex decision based on patient’s age, diagnosis, donor availability, and comorbidity.
1. Stem cell source.
a. Bone marrow.
b. “Mobilized” peripheral blood: growth factors are used alone (e.g., granulocyte-colony stimulating factor [G-CSF]) or in combination with chemotherapy (in autologous HCT) for “mobilization” of hematopoietic stem cells, which are collected by leukapheresis.
c. Cord blood: collected from the umbilical cord after delivery and cryopreserved.
2. Donor type.
a. Autologous: using one’s own stem cells.
b. Syngeneic: identical twin (no genetic donor/recipient disparity).
c. Allogeneic: donor with similar human leukocyte antigen (HLA) type.
i. Sibling donor: Statistical likelihood for an HLA-identical sibling is 25% per sibling.
ii. Unrelated donor: HLA-matched volunteer donor is identified through a database search.
3. Intensity of the preparative regimen.
a. Myeloablative: the regimen ablates the hematopoietic system and leads to transient but profound myelosuppression with pancytopenia.
b. Reduced-intensity: preparative regimen has reduced early toxicity and may therefore be outpatient based. The burden of disease
eradication is on ensuing immunologic graft-versus-tumor effects. Onset of typical post-HCT complications, such as GVHD and infections, may be delayed.
eradication is on ensuing immunologic graft-versus-tumor effects. Onset of typical post-HCT complications, such as GVHD and infections, may be delayed.
C. Transplant statistics: estimated number of HCTs: 45,000 to 50,000/year worldwide (approximately two-thirds autologous and one-third allogeneic HCTs).
D. Risk factors for posttransplant complications: age, intensity of the preparative regimen, type and stage of underlying disease, presence of comorbidities, and HLA-disparity between donor and recipient. Allogeneic HCT recipients have greater risk of transplant-related morbidity and mortality than autologous recipients.
E. Prognosis: highly variable and influenced by numerous risk factors related to the transplant procedure and to recurrent malignancy after surviving the transplant.
II. TRANSPLANT-RELATED COMPLICATIONS
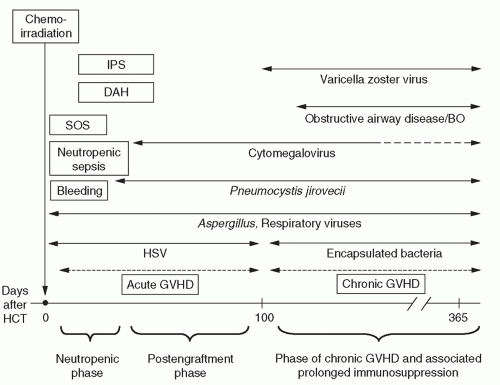
A. HCT-related morbidity and mortality can be categorized into four groups (Fig. 138-1).
1. Toxicity of the preparative regimen.
2. Infection.
3. Bleeding.
4. GVHD.
III. TOXICITY OF THE PREPARATIVE REGIMEN
A. General principles: cytotoxic chemotherapy with or without total body irradiation (TBI) may compromise the function of many organs. This toxicity occurs predominantly within the first 3 to 4 weeks of HCT and is less severe after reduced-intensity HCT.
1. Lung.
a. Etiology/pathophysiology.
i. Idiopathic pneumonia syndrome (IPS), a noninfectious inflammatory lung process that may be triggered by TBI and chemotherapeutic drugs (e.g., carmustine [BCNU], busulfan); median onset: 2 to 3 weeks after HCT. Contributing factors include release of inflammatory cytokines due to alloreactivity or sepsis; role of latent infections is controversial.
ii. Other common noninfectious pulmonary problems that may occur within 30 days posttransplant include diffuse alveolar hemorrhage (DAH), edema syndromes due to fluid overload or cyclophosphamide-induced cardiomyopathy, and sepsis with adult respiratory distress syndrome (ARDS).
b. Diagnosis.
i. Clinical presentation: fever, nonproductive cough, tachypnea, hypoxemia. Hemoptysis is an infrequent symptom of IPS or DAH.
ii. Laboratory and radiologic studies: diffuse or multifocal intraalveolar or interstitial infiltrates on chest radiography or computed tomography (CT). An increased alveolar-arterial oxygen gradient, a new restrictive pattern, or a diffusion-capacity abnormality is evidence for abnormal pulmonary physiology. Echocardiography may be useful to rule out cardiogenic pulmonary edema.
iii. Differential diagnosis: must distinguish noninfectious (IPS) from infectious causes (e.g., cytomegalovirus [CMV], bacterial, fungal) of diffuse pulmonary infiltrates. Localized parenchymal lung disease is usually due to infection. Diagnosis of IPS requires a negative bronchoalveolar lavage or lung biopsy with stains and cultures for bacteria, fungi, and viruses. Bronchiolitis obliterans (BO) can be related to chronic GVHD. Patients may also have pulmonary relapse of their underlying malignancy.
iv. Treatment: IPS treatment consists of supportive care, secondary infection prevention, empiric broad-spectrum antibiotics, and trial of diuretics. Bleeding disorders should be corrected. Although there are no prospective trials to support the use of high-dose corticosteroids, a small percentage of IPS patients respond to prednisone (1 to 2 mg/kg/d). Patients with BO frequently respond to corticosteroid therapy (1 mg/kg/d).
v. Prognosis: mortality of diffuse IPS after myeloablative HCT is 50% to 70%. Approximately 6% of patients who require mechanical ventilation after HCT survive for 30 days after extubation and are discharged; half of these survivors live for >2 years. The presence of either hemodynamic instability or sustained hepatic and renal failure further worsens their prognosis. Since
ongoing improvements in supportive care may improve outcome, treatment decisions have to be individualized. Therefore, aggressive management, including mechanical ventilation, to identify and treat reversible causes of respiratory failure is a reasonable initial approach for most HCT recipients with diffuse or multifocal pulmonary infiltrates.
ongoing improvements in supportive care may improve outcome, treatment decisions have to be individualized. Therefore, aggressive management, including mechanical ventilation, to identify and treat reversible causes of respiratory failure is a reasonable initial approach for most HCT recipients with diffuse or multifocal pulmonary infiltrates.
2. Gastrointestinal (GI) tract.
a. Etiology/pathophysiology: oral mucositis, esophagitis/gastritis, and diarrhea are commonly associated with chemoradiation and can be worsened by methotrexate given for GVHD prophylaxis. Mucositis places patients at high risk for aspiration and facilitates translocation of intestinal bacteria and sepsis.
b. Diagnosis.