(1)
Critical Care Medicine and Pain Medicine, Boston Children’s Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, MA, USA
Keywords
ECMOOxygenation indexPancreatitisARDSAlveolar-arterial gradientIntracranial pressureA 4-year-old with gram-negative sepsis develops increasing respiratory distress concomitant with antibiotic treatment and her admission to the ICU.
Case # 1: Acute Respiratory Distress Syndrome (ARDS)
Questions
- 1.
What do you think is going on? Why?
- 2.
How does endotoxin release promote ARDS? What are some of the vasoactive mediators involved?
- 3.
How does the release of leukotrienes, tumor necrosis factor (TNF), and prostaglandins involve the pulmonary circulation in ARDS? What implications are there for you as a critical care anesthesiologist? Why?
- 4.
Is there a role for surfactant instillation in such patients? Does surfactant work normally in patients with ARDS? Why/why not? What can you do about it?
- 5.
Will PEEP help? Why? What are the adverse effects of high ranges of PEEP? How can these effects be minimized?
- 6.
Under what circumstances would you consider extracorporeal membrane oxygenation (ECMO)? What about extracorporeal CO2 removal (ECCO2R)?
- 7.
What effects do ARDS have on shunt (venous admixture)? Are there any effects on Vd/Vt? What are they? At what phase of the disease process? What, if anything, can you do about it?
Case # 1: Acute Respiratory Distress Syndrome (ARDS)
Answers
- 1.
This child may be developing acute respiratory distress syndrome (ARDS) [1, 2, 3]. ARDS is characterized by the following:
- (a)
Acute onset of symptoms
- (b)
Severe respiratory failure with PaO2/FiO2 <200 mmHg regardless of positive end-expiratory pressure (PEEP) levels
- (c)
Chest X-ray that shows bilateral infiltrates
- (d)
Lack of clinical evidence that left ventricular (LV) failure is the etiology of the respiratory distress
- (a)
In addition, the deterioration of pulmonary function often is associated with a non-pulmonary clinical insult [4]. In pediatrics, the more commonly associated conditions are shock, sepsis, or drowning. Other associated conditions include massive transfusion, smoke inhalation, burns, or trauma [5].
- 2.
Since sepsis is associated with the development of ARDS, endotoxins released by bacteria, mediators released by inflammatory cells, and other compounds such as complement, products of disseminated intravascular coagulopathy (DIC), prostaglandins, and leukotrienes have all been evaluated for their role in the development of this clinical syndrome. Although the effects of these many mediators are interconnected, endotoxin has been shown to have several effects itself. Lipopolysaccharide from gram-negative bacteria has been shown to directly affect the integrity of the endothelium and also to stimulate macrophages to release tumor necrosis factor (TNF) and interleukin (IL)-1 [6]. One source of endotoxin in patients with sepsis and hypotension is thought to be the pulmonary capillary endothelium that is damaged. This damage is caused by the release of many of the mediators mentioned above. Once the integrity of the alveolar-capillary membrane is disrupted, a proteinaceous, hemorrhagic fluid enters the alveolar space.
- 3.
Another effect of some of these mediators, particularly products of arachidonic acid metabolism such as the leukotrienes and prostaglandins, is to contribute to the development of pulmonary hypertension. A cycle of pathology ensues. As hypoxia worsens, the pulmonary artery pressure rises further, and with continued release of the various mediators, pulmonary edema worsens, and reactivity of the pulmonary vessels to hypoxia also worsens. Although the role(s) of mediators in ARDS is being more and more well characterized, therapy directed toward these compounds is still investigational. Studies of the use of steroids have been disappointing. Steroid administration has not reversed the pathophysiology nor decreased mortality in ARDS [7, 8].
- 4.
In ARDS many of the pathophysiologic abnormalities are due to diminished activity of surfactant. Functional residual capacity (FRC) is reduced and lung compliance is decreased. Surfactant acts to stabilize alveoli. Surfactant keeps surface tension proportional to surface area, allowing smaller alveoli to remain inflated at the same transpulmonary pressure as larger alveoli. Without surfactant, smaller alveoli would empty into larger ones resulting in areas of collapse. In the lab, it has been shown that the surface activity of phospholipids from patients with ARDS is poor. Surfactant administration to preterm newborns with RDS, while not curative, has had some salutary effects on the course of the disease such as decreased mortality, decreased incidence of air leak, and improved oxygenation [5]. Unfortunately, administration of surfactant to patients with ARDS has not affected mortality.
- 5.
The goal of therapy for patients with ARDS is to maintain adequate oxygen delivery while minimizing the harm of the therapy directed toward achieving that oxygen delivery [9]. Loss of FRC is an important part of the pathophysiology in ARDS. Application of PEEP increases the FRC. The likely mechanism for the increase in FRC is recruitment of previously collapsed terminal alveoli. PEEP also improves the static compliance of the lung. The pulmonary effects of PEEP in patients with ARDS, then, are increased FRC and improved compliance resulting in increased PaO2 and decreased shunt (Qs/Qt ). The improved oxygenation is the result of better blood flow to ventilated alveoli. PEEP may also decrease cardiac output, however, primarily by decreasing venous return. At modest levels of PEEP, increasing intravascular volume may compensate for these deleterious cardiovascular effects. At some point, excessive PEEP will actually decrease oxygen delivery since the fall in cardiac output will exceed the increased oxygen content of the blood. Another deleterious effect of PEEP is the development of pulmonary edema. PEEP lowers pulmonary interstitial pressure, increasing the pressure gradient for an increase in extravascular lung water (EVLW). Inhaled nitric oxide (iNO), a pulmonary vasodilator, has been administered to patients with ARDS. Since pulmonary arterial hypertension is a large part of the pathophysiology of the syndrome, iNO could be an effective treatment. Because it would only be delivered to well-ventilated parts of the lung, iNO in theory could improve V/Q matching while it lowered PAP. In adult studies, iNO has indeed been shown to decrease peak airway pressure and Qs/Qt. There is an emerging experience with iNO administration to children with ARDS, but no controlled studies have documented improved survival. Fluid therapy for children with ARDS can have a significant effect on the course of the illness. While both crystalloid and colloid will increase the intravascular volume initially and both will eventually leak into the alveoli, there are differences. In general, less colloid will leak into the alveoli. The amount of colloid that leaks into the alveoli depends on the molecular weight of the colloid. Pentastarch leakage into alveoli was less than hetastarch in an animal model of septic shock. Blood administration has many advantages in these patients. Oxygen delivery is increased immediately, and cardiac output is increased as preload is augmented. In addition, the packed red blood cells (PRBCs) are much less likely to leave the vascular space in significant amounts compared with colloid molecules or the ions in crystalloid.
- 6.
While ECMO does not appear to offer advantages in the care of adults with ARDS, it may be of benefit to children with this syndrome. The criteria for institution of this therapy (ECMO is not a treatment) have changed over time, but some underlying considerations remain. Among the considerations for determining the suitability of a patient for ECMO are the severity of the lung disease, the reversibility of the lung disease, and the involvement of other organ systems. The severity can be judged using a variety of measures such as the oxygenation index (OI), the A-a gradient, and Qs/Qt. The OI (MAP × FiO × 100)/PaO2) has been used to evaluate possible ECMO candidates for some time. An OI > 40 was believed to represent > 90 % risk for mortality. Extracorporeal removal of CO2 (ECCO2R) has not been shown to benefit adults with ARDS and has not been used much at all in the care of children with ARDS.
Case # 2: Shock/Multi-organ System Failure
Questions
A 15-year-old girl is admitted to the ICU with a presumptive diagnosis of toxic shock syndrome. Her blood pressure is 64/20 mmHg, heart rate 142/min; she is intubated and mechanically ventilated. Her arterial blood gas (FiO2 = 1.0) is pH = 7.12, paO2 = 97 mmHg, and paCO2 = 32 mmHg.
How will you proceed? What do you think is going on? She has no urine output; Foley catheter is in place. You place a pulmonary artery catheter; pulmonary capillary wedge pressure (PCW) is 3 mmHg, and PA pressures are elevated at 42/22 mmHg. What now? Should you give sodium bicarbonate? Why not? Will it make the dopamine work better? How does that happen?
Case # 2: Shock/Multi-organ System Failure
Answers
This patient’s condition meets the criteria for shock. She has evidence of circulatory failure and inadequate tissue perfusion [10]. The clinical diagnosis of shock is supported by the following: tachycardia, hypotension, poor capillary refill, oliguria, decreased pulse pressure, and tachypnea. The shock is probably due to both hypovolemia and maldistribution of the circulating blood volume. Abnormal vasomotor tone will exacerbate the effects of preexisting hypovolemia. She may have inadequate preload due to a variety of factors: recent poor PO intake during the prodrome of the illness and loss of intravascular volume through capillary leak. The distributive aspect to shock in this case results from the endotoxins released in the syndrome of toxic shock. These mediators diminish sympathetic tone, and the resulting lowered systemic blood pressure contributes to impaired perfusion of organs. The patient is responding to decreased stroke volume with an increased heart rate, but with the low systemic blood pressure, perfusion will be inadequate. The first priority in this situation is to improve cardiac output [6]. Increasing preload should be done first.
A rapid infusion of isotonic IV fluid, 20 mL/kg, should begin the improvement. If the patient’s perfusion still is inadequate after 40–60 mL/kg of isotonic IV fluid, placement of a central venous line (CVL) should be considered to monitor preload more directly and also to support cardiac function. The arterial blood gas (ABG) shows a metabolic acidosis, severe hypoxemia, and slight hyperventilation. The patient has an alveolar to arterial (A-a) gradient of >500. The PAO2, the alveolar partial pressure of oxygen, can be calculated with the formula
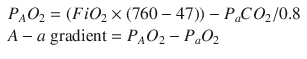
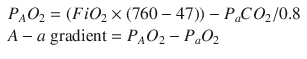
For this child, the calculation is
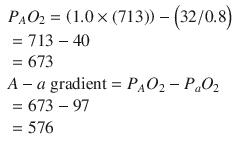
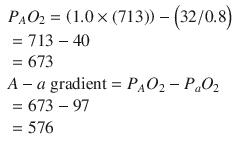
For a well person breathing room air, the calculation is
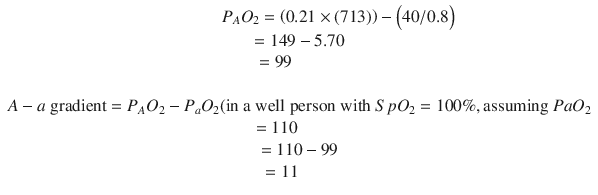
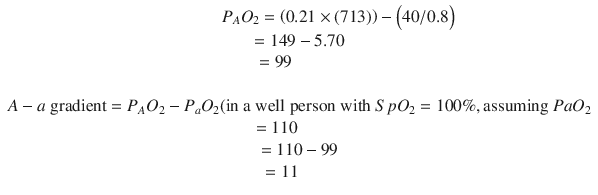
As preload is replenished and cardiac contractility is improved, there should be improvement in PaO2. If the patient’s A-a gradient does not improve as the circulatory disturbances are corrected, the ventilator setting should be adjusted. The patient may have better oxygenation with increased peak inspiratory pressures, a higher PEEP, or a change in the I/E ratio. Increases in mean airway pressure will generally increase oxygenation but will also affect venous return. As ventilator settings are adjusted, the cardiovascular parameters must be carefully observed for deterioration.
The acidosis is most likely due to impaired delivery of oxygen and substrates to the tissues, the pathophysiologic abnormality in shock states. Correction of the circulatory disturbances will lead to correction of the metabolic acidosis. If severe, acidosis will affect function of many enzymatic systems, including those responsible for myocardial performance. In cases of severe acidosis, drugs such as dopamine that enhance cardiac contractility have severely diminished effectiveness. With the pH seen in the ABG, dopamine is likely to have some effect and improve contractility. The pulmonary capillary wedge pressure (PCWP) reflects left atrial pressure, which, in turn, reflects LV end-diastolic pressure or preload. A PCWP of 3 mmHg in this case indicates a relatively low preload. The PA pressure of 42/22 mmHg is elevated. These numbers together indicate a lower preload with PA hypertension and increased RV afterload. Further treatment will be guided by the child’s clinical response to fluid therapy and dopamine, urine output, ABGs, and mixed venous blood gas analysis. If the patient continues to exhibit clinical signs of shock and continues with a base deficit of >6 mEq/L, administration of bicarbonate is indicated. Initial dosing of bicarbonate can be estimated with the formula mEq NaHCO3 = 0.3 × (weight in kg) × (base deficit).
Case # 3: Neurologic Intensive Care
Questions
A 5-year-old is admitted to the ICU with a small epidural hematoma and closed head injury. The neurosurgeon wants to observe him and only goes to the OR for evidence of acute deterioration.
- 1.
Do you agree with this? What is the basis for observing a patient with an epidural hematoma?
- 2.
The patient arrives from the ER with an IV of D5 1/2NS. Would you change it? Why? Under what circumstances would you reconstitute the IV fluids?
- 3.
The nurse asks you what position you would like the head of the bed to be in. Your answer? Why? What are the considerations in your answer? What is the optimal positioning for a patient with a space-occupying lesion and possible elevated intracranial pressure?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





