Coronary Artery Disease
Carlos A. Roldan
The history and physical examination are important in the initial assessment of patients with suspected or known coronary artery disease (CAD).
However, the history and physical examination in patients with CAD have several limitations. Up to 25% of elderly or diabetic patients can have a silent myocardial infarction (MI); the magnitude of symptoms does not correlate with the extent of CAD; with typical symptoms, <30% of admitted patients have an acute coronary syndrome (ACS); the predictive value for CAD of classic angina is approximately 50% in women; and most patients with an ACS have a normal physical examination.
Electrocardiography also has limitations in the diagnosis of CAD. Acute pericarditis, left ventricle (LV) aneurysm, and early repolarization may mimic acute ischemic injury. Also, ACS and many other conditions are associated with nonspecific ST-T abnormalities. In addition, approximately 15% to 20% of patients with ACS have a normal electrocardiogram (ECG).
Finally, of the more than 5 million Americans presenting to emergency rooms with chest pain, 85% do not have ACS, but 3% to 5% with acute MI (AMI) are discharged home.
Therefore, echocardiography (echo) is of important complementary value to the history and physical examination and ECG in the diagnosis and management of CAD.
Prevalence
CAD is the most prevalent clinical problem in adult cardiology; it affects >12 million Americans and is the leading cause of death in United States (1).
Echocardiography
Class I or Appropriate (Score 7 to 9) Indications for Resting and Stress Echocardiography in Coronary Artery Disease
Resting and stress echo play a very important role in the diagnosis, assessment of severity and prognosis, detection of complications, management, and follow-up of patients with chest pain, ACS (ST-segment elevation MI [STEMI]), non–ST-segment elevation MI (NSTEMI), unstable angina (USA)], chronic CAD, and with ischemic diastolic or systolic heart failure (1,2,3,4,5,6) (Tables 2.1 and 2.2).
Diagnosis of Coronary Artery Disease: Methods and Key Diagnostic Features
Two-dimensional and Real-time Three-dimensional Resting Echocardiography
Wall Motion and Coronary Artery Supply
Best Imaging Planes
Two-dimensional (2D) transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE) short-axis and apical four-, two-, and three-chamber views.
Wide-angle or full-volume real-time three-dimensional (RT3D) TTE or TEE four-chamber view (7).
Normal Wall Motion
Wall motion abnormalities are the sine qua non of myocardial ischemia or MI.
Abnormal resting LV wall motion is best defined by a <30% systolic endocardial thickening (best defined by
M-mode) and less specifically by <0.5-cm inward wall motion.
Table 2.1 Class I indications or appropriate criteria (score 7–9) for resting echocardiography in patients with suspected or known coronary artery disease
Patients with chest pain and nondiagnostic or uninterpretable ECG, shortness of breath, light-headedness, presyncope, or syncope.
Patient with chest pain and hemodynamic instability.
Patients without chest pain but with other features of an ischemic equivalent, abnormal ECG, or laboratory markers indicative of ongoing MI or ischemia.
Initial evaluation of ventricular systolic and diastolic function following a confirmed ACS.
Re-evaluation of ventricular systolic and diastolic function following ACS during recovery phase when results will help guide therapy.
Patients with known or suspected ischemic systolic or diastolic heart failure.
Re-evaluation of ischemic systolic or diastolic heart failure when a change in clinical status or cardiac exam has occurred.
Suspected post MI acute mitral regurgitation, ventricular septal defect, free wall rupture/tamponade, shock, right ventricular infarction, or thrombus.
Evaluation of ischemic mitral regurgitation to assess suitability for, and assist in planning of, valve repair or replacement (2D or RT3D TEE)
Suspected cardiovascular source of embolus in a patient with a large MI.
Suspected post MI pericarditis or Dressler syndrome.
ECG, electrocardiography; MI, myocardial infarction; ACS, acute coronary syndrome; 2D, two-dimensional; RT3D, real-time three-dimensional; TEE, transesophageal echocardiography.
Adapted from Douglas PS. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–1166.
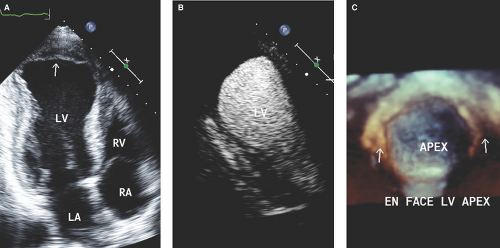
Each of the 16 or 17 (when the apical cap is included) LV wall segments is scored as follows: 1, normal or hyperkinetic; 2, hypokinetic; 3, akinetic; 4, dyskinetic; or 5, aneurysmal (diastolic deformation) (Fig. 2.1 and Table 2.3). Thus, a normal global wall motion score is 16 or 17, and a normal wall motion score index is 1 (4,5,8). The higher the score and index, the worse the extent and severity of ischemia or MI.
Coronary Artery Supply of the Left Ventricle
Basal, mid-, and apical anterior and basal, mid-, and apical anterior septum are supplied by the left anterior descending (LAD) artery (4,5) (Table 2.4; see Fig. 1.6).
Basal and mid-anterolateral and apical lateral segments are supplied by the LAD or left circumflex (LCX) artery.
Basal and mid-inferior walls and basal inferior septum are supplied by the right coronary artery (RCA).
The mid-inferior septum and apical inferior segments are supplied by the RCA or LAD artery.
Basal and mid-inferolateral segments are supplied by the RCA or LCX artery.
Coronary Artery Supply of the Right Ventricle
As for the LV, right ventricle (RV) wall motion is defined by a decreased systolic inward motion and less precisely by endocardial thickening.
Blood flow to the RV walls is provided predominantly by the RCA as follows (10):
Anterior and lateral walls by the acute marginal branch.
Inferior and inferoseptal walls by the posterior descending artery.
RV outflow tract anterolateral wall by the conus branch.
Stress Echocardiography
Best Imaging Planes
Indications
Stress echo is indicated for (i) the detection and assessment of location and extent of CAD in symptomatic and
asymptomatic patients or in patients with other abnormal or equivocal imaging studies, (ii) for assessment of ischemic or viable myocardium post ACS or post revascularization procedures, and (iii) for risk stratification (Table 2.2).
Table 2.2 Class I or appropriate indications (score 7–9) for stress echocardiography in patients with suspected or known coronary artery disease | ||||||
|---|---|---|---|---|---|---|
|
Key Diagnostic Features
An abnormal wall motion response to exercise or dobutamine demonstrates a decreased to absent inward motion in association with decreased (<30%) to no systolic endocardial thickening.
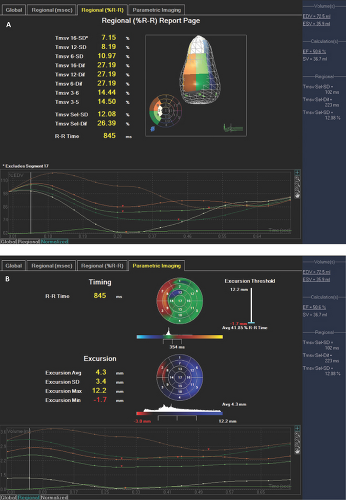
With RT3D imaging, resting and peak stress segmental and global wall motion can be visually assessed from multiple views, and for each wall segment, a time to end systole and extent of systolic excursion are graphically displayed.
Comparison of resting with peak stress wall motion results in four types of wall motion response and corresponding clinical scenarios: (i) absent or low likelihood of CAD, (ii) ischemia without MI, (iii) MI with ischemic viable myocardium (stunned or hibernating), or (iv) MI with nonviable myocardium (Table 2.5).
The diagnostic accuracy of stress echo in the detection of CAD increases as (i) the severity and extent of CAD increase, (ii) the severity of wall motion or number of asynergic segments increases, and (iii) the predicted maximal heart rate is achieved or exceeded.
Contrast Echocardiography
At least 20% of resting or stress 2D and RT3D echocardiograms are technically difficult or unable to visualize two or more contiguous LV segments in patients with suspected or known stable CAD or ACS, or in those with post MI mechanical complications.
Table 2.3 Scoring system for grading left ventricular wall motion
Score
Wall Motion
Systolic Wall Motion
Endocardial Thickening
1
Normal
Normal
Normal (>30%)
2
Hypokinesis
Reduced
Reduced (<30%)
3
Akinesis
Absent or reduced if dragged by other normal walls
Absent; thinning and hyperreflectance are common
4
Dyskinesis
Outward
Thinning and hyperreflectance in most cases
5
Aneurysmal
Outward. Diastolic deformity present
Absent and thinning
In patients with suboptimal studies, resting or stress contrast 2D or RT3D echo plays an important role in the diagnosis and management of patients with CAD.
Contrast 2D and RT3D echo are mainly approved for LV opacification and endocardial border definition, but they can also be used for enhancement of aortic
stenosis, tricuspid regurgitation, and mitral and pulmonary inflow Doppler signals (4,9,11).
The approved and most commonly used intravenous contrast agents contain microbubbles with thin and permeable shells of human serum albumin (Optison) or phospholipid (Definity) filled with a high-molecular-weight perfluorocarbon gas, which slows diffusion and dissolution within the bloodstream.
Resting or stress contrast 2D or RT3D echo results in a 20% to 25% increase in the number of segments with complete endocardial border visualization, a 30% to 35% increase in the number of good-quality studies, and a significantly improved intra- and interobserver agreement for assessment of global and regional LV systolic function:
Table 2.4 Left ventricular wall segments and corresponding coronary artery supply
Left Ventricular Wall Segment
Coronary Artery Supply
Basal, mid, and apical anterior
LAD artery
Basal, mid, and apical anterior septum
LAD artery
Apical lateral
LAD artery
Basal and mid-anterolateral
LAD or LCX artery
Basal and mid-inferior
RCA
Basal and mid-inferior septum
RCA
Apical inferior
RCA or LAD artery
Basal and mid-inferolateral
LCX artery
Right Ventricular Wall Segment
Right Coronary Artery Branch
Anterior and lateral walls
Acute marginal branch
Inferior and inferoseptal walls
Posterior descending artery
RVOT anterolateral wall
Conus branch
LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery, RVOT, right ventricular outflow tract.
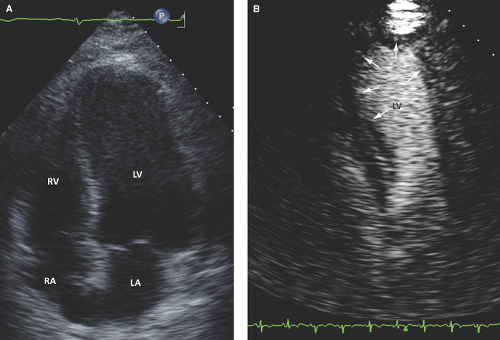
Therefore, the improved diagnostic quality of contrast studies results in improved assessment of LV structure, wall motion and thickening, volumes, and function (Fig. 2.3).
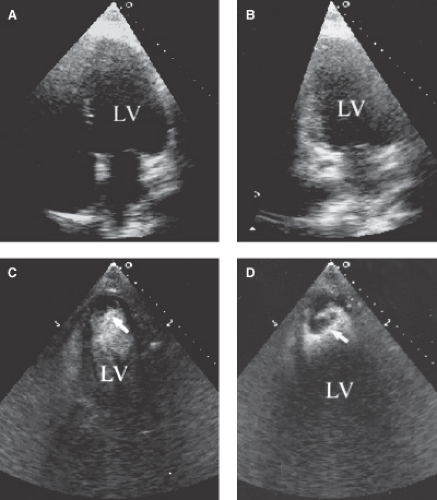
Increased diagnostic accuracy in the detection of LV apical thrombus, aneurysm, pseudoaneurysm, and myocardial rupture (Figs. 2.4 and 2.5).
Contrast echo also allows identification of the presence and extent of microvascular disease by detecting a myocardial perfusion defect in an open epicardial coronary artery with slow or <3 Thrombolysis in Myocardial Infarction (TIMI) flow in AMI patients post percutaneous coronary intervention (PCI) or thrombolysis therapy. However, this is not an approved indication.
Table 2.5 Patterns of left ventricular wall motion on stress echocardiography and clinical implications
Wall Motion at Rest
Wall Motion at Peak Stress
Clinical Implication
Normal wall excursion and endocardial thickening
Hyperdynamic and symmetric wall thickening
Absent or very low likelihood of coronary artery disease
Normal wall excursion and endocardial thickening
Hypokinetic, akinetic, uncommonly dyskinetic
Ischemia without MI
Wall hypokinesis or akinesis with partial or full endocardial thickening
Augmented, hypokinetic, akinetic or dyskinetic
MI with viable stunned (if augmented), ischemic (if worsens), or hibernating myocardium (if a biphasic response noted)
Akinetic and thinned wall
Akinetic or dyskinetic
Transmural MI and no viability
MI, myocardial infarction.
Also, contrast echo can predict LV remodeling post MI (defined as >20% increase in LV end-diastolic volume). A TIMI flow grade <3 and a myocardial contrast defect >25% are independent predictors of LV remodeling (12).
Contrast agents are administered by intravenous push or continuous infusion with a recommended rate of bolus injection of 0.5 to 1.0 mL/second (most commonly used is 2 to 4 mL per injection) followed by a slow saline flush of 2 to 3 mL over 3 to 5 seconds.
Imaging should be performed using a low mechanical index of 0.15 to 0.3.
Although cavity-enhancing contrast agents have shown to be safe with no evidence of deaths or MIs within 30 minutes, 60 minutes, 24 hours, or 30 days as compared to patients undergoing noncontrasted echo (11,13), they are contraindicated in patients with cardiac shunts; hypersensitivity to perfluorocarbon; and hypersensitivity to blood, blood products, or albumin:
Also, contrast agents should be used with caution in patients with ACS, decompensated heart failure, serious ventricular arrhythmias or those at high risk for arrhythmias, respiratory failure, severe emphysema, pulmonary emboli, or pulmonary hypertension. If used in these patients, monitoring of vital signs, electrocardiography, and cutaneous oxygen saturation for at least 30 minutes is recommended.
As well, at least verbal consent should be obtained in all patients, and documentation of the indication and discussion with patients of the benefits and risks of the procedure is recommended.
Assessment of Myocardial Viability
Dobutamine echo is the most commonly used technique for assessment of myocardial viability (2,3,4,5,14) (Table 2.5).
A resting hypokinetic or akinetic segment that improves in wall motion and endocardial thickening with low-dose dobutamine (≤10 μg/kg/minute) but deteriorates at a higher dose (“biphasic response”) indicates viable ischemic myocardium with a flow-limiting coronary artery stenosis.
A resting hypokinetic or akinetic wall that shows sustained (low-dose and high-dose) improvement in wall motion and endocardial thickening indicates viable nonischemic myocardium with a non–flow-limiting coronary artery stenosis.
An akinetic, thin, and hyperreflectant segment with no change with dobutamine indicates nonviable myocardium.
Resting Echocardiography in Patients with Suspected Acute Coronary Syndromes
Key Diagnostic Features
Segmental wall motion abnormalities occur within 30 minutes of a coronary artery occlusion and before ischemic ECG changes and elevation of myocardial isoenzymes.
In patients with chest pain, a new or worsening (transient or persistent) wall motion abnormality indicates myocardial ischemia or AMI (new, extension, or expansion) with high sensitivity, specificity, and predictive value (2,3,4,15).
Resting echo detects wall motion abnormalities in 90% to 100% of patients with STEMI and identifies the culprit coronary artery (concordance with angiography) in ≥90% of patients with STEMI, 75% to 85% of those with NSTEMI, and 20% of patients with USA (15). Thus, absence of wall motion abnormalities during chest pain indicates a low likelihood of AMI or a small branch ischemic territory.
Key Prognostic Features
In patients with USA, new or worsening LV systolic dysfunction predicts severe CAD with extensive areas of jeopardized ischemic myocardium.
A wall motion score index >1.5 or four or more abnormal segments in the distribution of an MI-related artery or in remote areas are highly predictive of large MI or multivessel CAD, respectively.
A wall motion score index >1.5 or left ventricular ejection fraction (LVEF) ≤40% indicates higher in-hospital post MI angina, MI extension or expansion, post MI mechanical complications, ventricular arrhythmias, heart failure, cardiogenic shock, and death.
Patients with moderate to severe LV systolic dysfunction have three times higher mortality than those with normal LV function, even with a similar extent of CAD.
Thus, resting echo in patients with ACS identifies those who would benefit from aggressive medical therapy, urgent cardiac catheterization, and percutaneous or surgical coronary revascularization.
Resting Echocardiography for Post Myocardial Infarction Patients
Key Diagnostic Features
Salvaged or Jeopardized Myocardium
The comparison of resting wall motion abnormalities or perfusion defects by myocardial contrast echo before and after reperfusion interventions determines the extent of salvaged myocardium and the risk of recurrent ischemia or MI.
Post reperfusion therapy, myocardial stunning can resolve within 3 to 5 days. Thus, improvement of LV wall motion and function on predischarge echo after an anterior MI may help in the decision of long-term anticoagulation in these patients.
Persistent significant wall motion abnormalities with thinning or hyperreflectance, LV aneurysm, or LVEF ≤40% suggest large AMI. In these patients, aggressive afterload reduction therapy for LV remodeling is needed and anticoagulation may be considered.
Post Myocardial Infarction Ischemia, Extension, or Expansion
Post MI angina: Transient or persistent worsening of wall motion abnormalities in the MI or non-MI–related artery distribution without re-elevation of myocardial isoenzymes.
Post MI extension: New or worse wall motion abnormalities with re-elevation of myocardial isoenzymes.
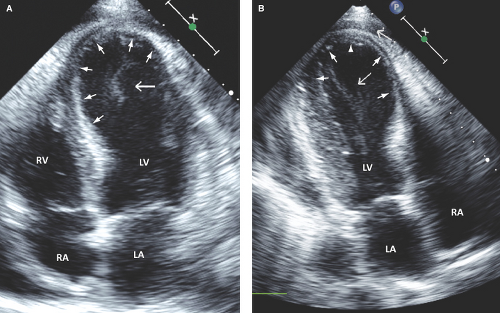
Post MI expansion: Increased area of wall thinning and wall stress in the infarct zone; it is progressive within 7 days post MI and may lead to aneurysm and/or thrombus formation, wall rupture, recurrent ischemia, heart failure, and ventricular arrhythmias (Fig. 2.1).
Right Ventricular Infarction
RV MI occurs in 25% to 30% of patients with posteroinferior MI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree