Links: Etiology | Pink Puffer | Blue Bloater | Hx | S/s & Dx | Labs | Tx | Stages | Ddx | Emphysema | Bronchiectasis | O2 therapy | Chronic Bronchitis | Tx of exacerbations | Step-Care Stages | Meds |
 Characterized by the progressive development of airflow limitation that is not fully reversible. 4th leading cause of death in the USA in age 65-84yo.
Characterized by the progressive development of airflow limitation that is not fully reversible. 4th leading cause of death in the USA in age 65-84yo.
• COPD is the third leading cause of death globally, and one of every four men and one of every six women will develop COPD if they live to be 95 years old 18th Annual Congress of the European Respiratory Society 2008;October)…..The risk of developing COPD was 3.8-fold higher in smokers than nonsmokers. A 19th century epidemic, COPD incidence and cigarette use are parallel.
• 15 to 25% of adults >40yo may have stage 1 mild COPD or higher and that prevalence is higher in smokers and ex-smokers, those over age 40 (Am J Respir Crit Care Med 2007;176:532-555)…several genes influence the development, but >80% of pt’s with COPD are or were smokers.
• 23% of Americans with airway obstruction have never smoked according to analysis of data from the Third National Health and Nutrition Examination Survey (NHANES) (Am J Med 2005;118:1364-1372) (mechanism unclear, may be completely different).
• In an study of 1,800 autopsies, the prevalence of emphysema judged to be advanced in 0% in nonsmokers, 12% in smokers of <1PPD, and 19% in those who consumed >1PPD (Clin Chest Med 2000;21:67). Decr FEV1 nl decline is 30-35 ml/yr, smokers lose 70-150 ml/yr.
• COPD in 12% of adults age >55yo…. affecting 5–15% of all adults in industrialised countries (Am J Respir Crit Care Med 2001;163:1256–76). The terms COPD, chronic obstructive lung disease and chronic airflow obstruction (CAO) are synonyms used to describe the minimally reversible airflow obstruction caused by chronic bronchitis, emphysema, or both.
• A study from Nepal shows that smoke from organic matter used as fuel – known as biomass smoke — worsens lung function, even in young adults (Eur Respir J 2012;online May 3rd)…..In 1,392 young adults from Nepal, FEV1, FEV1/FVC, and FEF25-75 were significantly lower in those who used biomass fuels, even after adjusting for potential confounders, but there was no significant association between FVC and biomass use…..The prevalence of airflow obstruction was significantly higher with biomass smoke exposure (8.1% vs 3.6%).
ICD-9 Codes:
490 Bronchitis, not specified as acute or chronic
491.0 Simple chronic bronchitis
491.1 Mucopurulent chronic bronchitis
491.2 Obstructive chronic bronchitis
491.21 With acute exacerbation
492 Emphysema
Etiology: Chronic bronchitis, emphysema, asthma, bronchiectasis, CF, BO all increase inflammation that may lead to airway obstruction. Smoking or passive smoking are major risk factors. Environmental pollutants, occupational dust/ chemicals, severe childhood respiratory infections or cooking in confined spaces in developing counties is common (indoor air pollution). Alpha-1 Antitrypsin def is seen in <2% of COPD pt’s. Chronic HIV or hepatitis C infection, low body mass, and low intake of dietary antioxidants may be cofactors in some cigarette smokers. Chronic malnutrition (first observed in Warsaw ghetto during World War II). Low birth wt infants are also at risk due to poor nutrition in fetal life. Anorexia nervosa causes a loss of lung tissue and decreased diffusing capacity, resulting in changes that resemble emphysema (Am J Respir Crit Care Med 2004;170:748-752).
• Eating too much cured meats (bacon, lunch meats and sausage >14 times a month) may increase the risk of COPD by 80% according to NHANES data (Nitrites are pro-oxidants that create reactive nitrogen species, they generate reactive nitrogen species that can cause damage to the lung) (Am J Respir Crit Care Med 2007;175:798-804)…these pt’s frequently have lower intakes of vitamin C, beta-carotene, fish, fruits, vegetables, and vitamin or mineral supplements.
• A protein microarray platform (PMP) can give an indication of risk and outcome in pt’s with COPD (Thorax 2007;62:595-601)…an elevated level of metalloproteinase-9 (MMP-9, a suspected causative factor for emphysema) showed the strongest association with FEV1 and carbon monoxide transfer factor.
• Neutrophil elastase is also elevated in COPD pt’s. Poor airway function in early infancy should be recognized as an independent risk factor for COPD in young adults according to the results of a nonselective longitudinal cohort study with 123 participants in Arizona (Lancet. 2007;370:717-719, 758-764).
• Inflammation is involved in the response to deep inspiration in pt’s with asthma, but this does not appear to be the case in those with COPD (Am J Respir Crit Care Med 2007;176:109-111,121-128).
• A preventable and treatable disease with some significant extrapulmonary effects (Gold Summary. Am J Respir Crit Care Med 2007;176:532-555).
Occupational Irritants: Increase the risk of COPD. Agricultural worker (endotoxin), coal miner (coal dust), concrete worker (mineral dust), construction worker (dust), gold miner (silica), hard rock miner (mineral dust), rubber worker (industrial chemicals). It is estimated by the WHO that by the year 2030, COPD will rise from its current ranking as fifth most common cause of death to the fourth most common cause of death, behind only ischemic heart disease, cerebrovascular disease, and HIV/AIDS (PLoS Medicine 2007;5:e112).
PP: It is characterized by chronic inflammation throughout the airways, parenchyma and pulmonary vasculature leading to irreversible fibrosis and narrowing of the small airways.
• Small airways are the major site of airway obstruction in pt’s with COPD and that these are compromised by the presence of inflammatory exudates (NEJM 2004;350:2635-2637,2645-2653), the inflammatory response in COPD appears to increase with the severity of disease and therefore with time, rather than burning out (which is seen in RA and interstitial lung disease).
• Usually no sx’s until FEV1 has fallen to ~50%. Lung colonization by pneumocystis is associated with more severe airflow obstruction (inflammation) in pt’s with COPD (Am J Respir Crit Care Med 2004;170:408-413).
• In ex-smokers with COPD, bacterial colonization is associated with neutrophilic airway lumen inflammation (Am J Respir Crit Care Med 2006;173:991-998).
• The rate of COPD exacerbations in pt’s with GERD sx’s is twice as high as in those without GERD (Chest. 2006;130:1096-1101)…may be microaspiration of gastric contents and/or vagal irritation.
• The etiology for acute exacerbations for COPD may be different in different localities and local data are thus important for guiding the choice of therapy (Chest 2007;131:44-52)….the bacteria most commonly recovered from sputum cultures were H. influenzae (13%), P. aeruginosa (6%), S. pneumoniae (5.5%), and M. catarrhalis (4.2%) in one study…..most S. pneumoniae isolates were at least intermediately resistant to penicillin, and 10.1% of H. influenzae showed beta-lactamase activity.
• A study provides evidence that single nucleotide polymorphisms of a particular gene (ADAM33, a disintegrin and metalloproteinase) affect a smoker’s risk of developing COPD (BMC Respir Res 2009;10:online March 12)…..located on chromosome 20p13, it has been shown in previous studies to be associated with asthma and bronchial hyperresponsiveness……All five SNPs (Q-1, S1, S2, V-1 and V4) were associated with COPD, and the S1 polymorphism had the strongest degree of association with COPD and pulmonary function abnormalities.
• Researchers found that living in cold, humid river valleys may exacerbate the symptoms of COPD (European Respiratory Society’s annual conference in Amsterdam. 2011. October 6)……”As a result of this unique combination of weather and climate, toxic particles and pollutants which would otherwise be small enough to be inhaled but subsequently exhaled become attached to droplets and are then retained within the lung causing exacerbation of symptoms.”
2 types: Same tx. These pt’s with COPD usually have a smoking history of at least 20 pack-years.
1. Pink Puffer: Emphysematous, thin, dyspneic, decr PaCO2, PO2 may be ok at rest, but decr O2 sat with exercise, wants O2 (but PCO2 often too high and must be cautious if give it), very hyperinflated. No sputum. Emphysema |
2. Blue Bloaters: Bronchitic (85% of COPD pt’s), obese, not dyspneic but significant coughing, incr PaCO2 (“retainers”), O2 low at rest, improved O2 SATs with exercise, needs O2 Rx (as gets cor pulmonale), less hyperinflated. Smoking is the primary etiology. But can be industrial related….Due prolonged exposure of nonspecific irritants to the tracheobronchial tree and is characterized by hypersecretion of mucus and structural changes in the bronchi, including inflammation, metaplasia of the epithelium and enlargement of the mucous glands. Hypoxia is less marked than in the bronchitic form of COPD.
Key Hx: age started smoking (even passive) & amounts, FHx, environmental factors. Pattern of sx development, cough, wheeze, DOE, frequency of acute chest illness. Impact on pt’s lifestyle. Comorbidity (heart, rheumatic dz).
S/s: Links: Pink Puffer | Blue Bloater | Typically present with cough, sputum production and dyspnea on exertion. Hyperresonance percussion, decr cardiac dullness, decr breath sounds, diaphragm moves <2cm, sub-abd PMI. Many get orthopnea soon after reclining (Vs pt’s with CHF, in whom orthopnea typically occurs hours after reclining, when fluid mobilizes from the lower extremities). Progressive inactivity to avoid dyspnea leads to physical deconditioning. Pt’s with more advanced disease may have pursed lip breathing or postures that relieve dyspnea (e.g., leaning forward against outstretched palms). The presence of significant edema may indicate right-sided heart failure and cor pulmonale in pt’s with pulmonary HTN from severe long-standing COPD.
• Women with COPD fare worse than men both in terms of the severity of their disease and their quality of life (ATS Conference. May 22, 2006).
• Facial wrinkling in smokers may be associated with COPD (Thorax. Posted online June 14, 2006) (reduced FEV1 and higher risks for COPD even after adjustment for age and sun exposure)…..despite the increased risk for wrinkles associated with smoking in the above study, only 27% of smokers with more than 50 pack-years of tobacco history had significant wrinkles.
• Independent of smoking, chronic cough and phlegm (an early marker) in young adults are strong predictors of increased risk of developing COPD (Am J Respir Crit Care Med. 2007;175:2-3).
• COPD erodes pt’s’ mental health, to a greater degree among women than men (Chest 2007;132:148-155)….the overall prevalence of psychiatric disorders at 49% was about 3 times higher than among the general population. Anxiety disorders were more common than mood disorders (49% versus 17%, respectively).
• Patients with COPD have disrupted coordination of the respiratory cycle with swallowing, which could increase the risk for aspiration according to a prospective study (Am J Respir Crit Care Med. 2009;179:559–565).
GERD: More than half of pt’s with advanced COPD have GERD (Chest 2007;131:1666-1671)….unclear if tx of GERD improves the clinical course of COPD. A study suggest that symptoms of GERD are an important factor associated with exacerbations in COPD (Thorax 2008;63:951-955)…..The incidence of COPD exacerbation was significantly higher in patients with GERD symptoms than in those without symptoms (relative risk 1.93; p < 0.01).
The typical ECG changes in COPD are: (1) prominent P waves in leads II, III and aVF;
(2) rightward shift of the QRS axis in the frontal plane (negative lead I and positive aVF);
(3) poor progression of the R wave in the precordial leads;
(4) low voltage of the QRS complexes especially in the left praecordial leads, and (5) the “lead I sign”(Chest. 2011;140:4). In patients with COPD, the frontal plane P, QRS and T wave axes are not infrequently all directed at around +90-. These three vectors are therefore directed either precisely or almost perpendicular to the standard lead I axis. As a result of this, lead I reflects either absent or very low amplitude P, QRS,T wave complexes giving the appearance of a minimally disturbed baseline. This ECG phenomenon is known as the “lead I sign”.
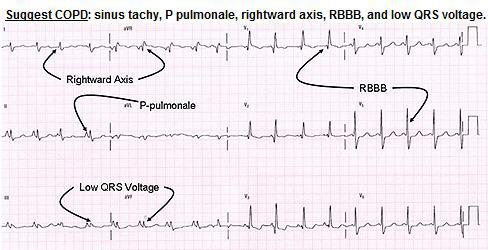 Cardiac: Measures of left-ventricular filling and cardiac output, but not LV ejection fraction, were inversely related to degree of emphysema and airflow obstruction in a population without clinical heart disease or severe lung disease drawn from the main cohort of the community-based Multi-Ethnic Study of Atherosclerosis (MESA) (NEJM 2010;362:267-268)…..suggesting that “subclinical hemodynamic changes occur with mild emphysema and airflow obstruction.”…..The findings were independent of whether participants were nonsmokers or had ever smoked.
Cardiac: Measures of left-ventricular filling and cardiac output, but not LV ejection fraction, were inversely related to degree of emphysema and airflow obstruction in a population without clinical heart disease or severe lung disease drawn from the main cohort of the community-based Multi-Ethnic Study of Atherosclerosis (MESA) (NEJM 2010;362:267-268)…..suggesting that “subclinical hemodynamic changes occur with mild emphysema and airflow obstruction.”…..The findings were independent of whether participants were nonsmokers or had ever smoked.
• Certain comorbidities common among people with COPD are independently associated with a higher likelihood of death according to data on 1664 patients followed for 51 months (Am J Respir Crit Care Med. Published online May 4, 2012)……The 12 comorbidities with the most robust association with increased death were cancers of the lung, pancreas, esophagus, and breast, followed by pulmonary fibrosis, atrial fibrillation/flutter, congestive heart failure, coronary artery disease, gastric/duodenal ulcers, liver cirrhosis, diabetes with neuropathy, and anxiety……Nonsurvivors had a higher number of comorbidities (6.5 ± 3.8) compared with survivors (5.8 ± 3.3 comorbidities).
Exacerbations: 3 key clinical criteria:
Incr sputum production & purulence and worsening dyspnea, other minor (URI in past 5d, incr wheeze, incr cough, fever w/o apparent cause, incr HR >20% over baseline). Also defined as two consecutive days, of an increase in two “major” sx’s, including dyspnea and sputum purulence, or an increase in one “major” and one “minor” sx (wheeze, sore throat, cough, and cold sx’s).
Dx: H&P (cough, sputum, dyspnea) confirmed with PFT’s (Spirometry | Obstructive |). See labs below. In general, a FEV1/FVC ratio of <70% of the predicted value suggests COPD. Flow volume loops has preserved insp but hypotenuse of the triangle has an incr angle shape (nl is a triangle on top of a ½ circle, concave). Stages: FEV6 may be a good surrogate for the FVC in the elderly as easier to get. The definition of airflow obstruction used by most clinical guidelines (FEV1/FVC <0.70) may lead to substantial overdiagnosis of COPD in older patients seen in primary care settings (Eur Respir J 2008;32:945-952)(91.2% specificity in younger pt’s drops to 82.0% in older pt’s).
Key Considerations in the dx: typically has a mid-life onset, slowly progressive sx’s, dyspnea, largely irreversible airflow limitation (asthma is typically reversible).
Chronic Cough: present intermittently or every day. Often present throughout the day, only seldom soley nocturnal.
Chronic Sputum Production: any pattern may indicate COPD.
Dyspnea: progressive, persistent (present every day), worse with exercise, worse with respiratory infections. The words used to describe the sensation of breathlessness are very different between people with and without COPD according to a study on 94 pt’s (Chest 2008;134:489-496)…..People without COPD used fewer words and more “physical” kinds of words (e.g., short of breath, breathing faster, working harder). People with COPD tended to use a greater variety of descriptions that were more emotional (e.g., frightening, worried, suffocating)…..the sensation of breathlessness described by people without lung disease is totally different to the sensation experienced by people with chronic lung disease…..the sensation of breathlessness includes both physical and affective (emotional) components, and in many ways the sensation of breathlessness is very similar to the sensation of pain.
Acute Bronchitis: repeated episodes. Exacerbations of COPD cluster together in time according to the results of a cohort study (Am J Respir Crit Care Med. 2009;179:335-336, 369-374)…..27% were followed by a second recurrent exacerbation within 8 weeks, and approximately one third of exacerbations were recurrent exacerbations……Initial exacerbations were not as severe as isolated events, but they were not less likely to be treated, so that undertreatment of initial events was not a plausible explanation for exacerbation recurrence……”At the very least, clinicians should now be aware that their patients with COPD who experience an exacerbation may be particularly ‘brittle’ during a subsequent 8-week period.”
History of exposure to risk factors: tobacco smoke. Occupational dust & chemicals. Smoke from home cooking and heating fuels. If young age (<45yo) or a strong FHx of COPD screen for Alpha-1 Antitrypsin deficiency…..testing should be performed in select pt’s (COPD in never-smokers, idiopathic cirrhosis, FHx of ±1-antitrypsin deficiency, predominantly lower lung emphysema, “premature” COPD, and refractory asthma at a young age).
COPD assessment test (CAT):
CAT score: The CAT questionnaire contains 8 items. Patients read the two statements for each item, which describe the best and worst scenario, and decide where on the scale (0-5) they fit. Scores for each of the 8 items are summed to give an overall score (minimum 0, maximum 40). A higher score indicates a worse health status.
I never cough 0 1 2 3 4 5 I cough all the time.
I have no phlegm (mucus) in my chest at all 0 1 2 3 4 5 My chest is completely full of phlegm (mucus).
My chest does not feel tight at all 0 1 2 3 4 5 My chest feels very tight.
When I walk up a hill or one flight of stairs I am not breathless 0 1 2 3 4 5 When I walk up a hill or one flight of stairs I am very breathless.
I am not limited doing any activities at home 0 1 2 3 4 5 I am very limited doing activities at home.
I am confident leaving my home despite my lung condition 0 1 2 3 4 5 I am not at all confident leaving my home because of my lung condition.
I sleep soundly 0 1 2 3 4 5 I don’t sleep soundly because of my lung condition.
I have lots of energy 0 1 2 3 4 5 I have no energy at all
<10 = Low. –> Smoking cessation. Annual influenza vaccination. Reduce exposure to exacerbation risk factors. Therapy as warranted by further clinical assessment.
10–20 = Medium. –> Patient has room for improvement. In addition to the guidance provided for patients with low impact CAT scores, you could consider: Reviewing maintenance therapy – is it optimal? Referral for pulmonary rehabilitation. Ensuring best approaches to minimising and managing exacerbations. Reviewing aggravating factors – is the patient still smoking?
21–30 = High. –> Patient has significant room for improvement. In addition to the guidance for patients with low and medium impact CAT scores, you could consider: Referral to specialist care (if you are in general practice). Additional pharmacological treatments.
>30 = Very High.
Scores obtained at exacerbation with the COPD assessment test (CAT) are a reliable indicator of exacerbation severity and can also be used to model recovery (Am J Respir Crit Care Med. Published online January 27, 2012)….Frequent exacerbators (=2 exacerbations per year) had significantly higher mean baseline CAT scores compared with infrequent exacerbators (<2 exacerbations per year; 19.5 ± 6.6 vs 16.8 ± 8.0; P = .025)…..At exacerbation, CAT scores rose significantly from the baseline value (from 19.4 ± 6.8 to 24.1 ± 7.3; P < .001), and this change in CAT score was significantly, but weakly, related to change in C-reactive protein (P = .008), but there was no significant change in fibrinogen (P = .351).
• Changes in the parameters of lung function before and after just 1 exacerbation of COPD underscore the impact of such exacerbations on lung function decline (European Respiratory Society (ERS) 2012 Annual Congress: Abstract 194OC. Presented September 2, 2012)…..the average patient with COPD is likely to experience 1 to 3 exacerbations a year…..The mean prebronchodilator FEV1 was –27.5 mL/year (range, –101.1 to 49.5 mL/year); after the exacerbation, this increased to –48.7 mL/year (range, –134.1 to 22.2 mL/year; P = .0006).
GOLD Assessment System to Guide Therapy of COPD Patients:
(Am J Respir Crit Care Med 2012 Aug 9;e-pub ahead of print)….To classify patients, the following steps are required:
– Determine risk, using either FEV1 or frequency of exacerbations.
– Assess symptoms, using either the Modified British Medical Research Council questionnaire on breathlessness or the COPD Assessment Test.
– Assign the patient to one of four severity groups, based on risk and symptoms.
– Treatment recommendations are provided for each of the four groups. Inhaled steroids are reserved for patients with either =2 exacerbations annually or FEV1 <50% of predicted.
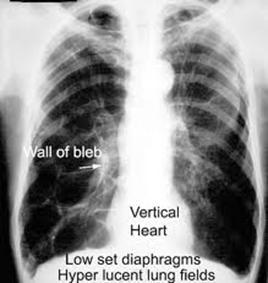
CXR: seldom diagnostic, but helps exclude other causes. CXR usually is abnormal in pt’s with severe COPD but may not show changes in up to one half of pt’s with moderate disease. May see emphysema, overdistension –> flat diaphragm, decr retrosternal airspace, narrow heart shadow. Increased basilar markings (“dirty lungs”) sometimes are visible on CXR in pt’s with chronic bronchitis, and isolated bullae may be seen in pt’s with emphysema. A CT scan of the chest should not be used routinely to dx COPD.
Spirometry: see obstruction –> decr VC & FEV1, also with evidence of airtrapping/ hyperinflation –> incr RV & FRC). May see decr DLCO (check if DOE out of proportion to PFT’s). If pt has a DLCO >36% and baseline O2 sat of <95%, will likely get desaturation during exercise (Arch Intern Med 2001;161:732). Testing peak expiratory flow rate (<80% of predicted) is a good means of detecting COPD in a community setting (BMJ 2003;327:653-4). Dyspneic COPD pt’s should be given a trial of bronchodilators even if PFT’s show that they do not manifest significant bronchodilation, because bronchodilator responsiveness may vary over time (Thorax 2003;58:659). (Spirometry) Although guidelines recommend that a COPD dx be confirmed with spirometry, new research suggests that such testing is only performed in 32% of cases (Chest 2007;132:403-409)….inexpensive, quick, and painless procedure, which is necessary to confirm a COPD dx. 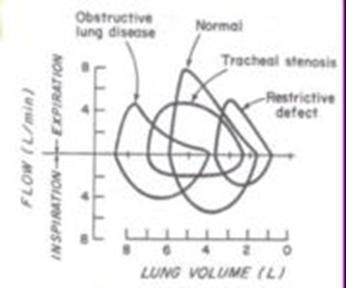
Screening: The USPSTF does not recommend routine screening for COPD with spirometry (Ann Intern Med. Published online March 3, 2008)….Spirometry is likely to identify a predominance of patients with mild to moderate airflow obstruction COPD who would not receive any additional health benefit if described as having COPD. Many patients would need screening to identity 1 person with COPD to avoid a single exacerbation. Evidence to date has not shown that spirometry independently improves smoking cessation rates. In the general US population, approximately 1 in 14 adults has objectively measured airflow obstruction consistent with COPD….Early pharmacologic tx, O2 therapy, and pulmonary rehabilitation for COPD may help sx’s, but none are associated with any significant decreased morbidity and mortality….Vaccination against influenza and pneumococcus are associated decreased exacerbations, but not with decreased morbidity and mortality from COPD.
ABG: not needed if only stage 1 airflow obstruction –> decr FEV>50% predicted). Check on all pt’s if have FEV1 <40% or signs of respiratory failure or R heart failure (edema). Recommended to rule out significant respiratory failure in pt’s with more severe disease….indicated by a PaO2 <60 mmHg (8 kPa) (= hypoxemia) with or without a PaCO2 >50 mmHg (6.7 kPa) (hypercapnia) while breathing air at sea level.
CRP: Levels of CRP and other inflammatory markers are elevated in pt’s with COPD and can be useful in predicting functional outcomes (Thorax 2006;61:10-28)….CRP levels are increased in pt’s with COPD 9-fold compared with control subjects. The report also shows that CRP levels are lower in pt’s treated with inhaled corticosteroids compared with untreated pt’s.
Procalcitonin: Using procalcitonin as a biomarker for exacerbations of COPD may help to specifically manage individual pt’s and reduce their overall exposure to antibiotics (Chest 2007;131:9-19) (reduced antibiotic prescriptions compared to standard therapy (40% versus 72%).
Serum amyloid A levels predict the severity of acute exacerbations in patients with COPD (Am J Respir Crit Care Med 2008;177:269-278)…..A serum amyloid A level of 12.5 mg/L provided 87% sensitivity and 92% NPV for severe acute exacerbations of COPD, compared with a 54% sensitivity and 79% negative predictive value for CRP…..A doubling in the level of serum amyloid A was 100% sensitive and 44% specific in predicting severe acute exacerbations of COPD, whereas a doubling in the level of CRP was 80% sensitive and 44% specific.
Bacteria: Researchers identified two distinct patterns of Pseudomonas aeruginosa colonization in patients with COPD — short-term colonization followed by clearance, and long-term, persistent carriage (Am J Respir Crit Care Med 2008;177:853-860)…..”The challenge is distinguishing those situations where P. aeruginosa is causing infection from those situations where the organism is simply a colonizer and another virus or bacterium is causing the exacerbation……Right now there is not a reliable way to make this distinction so clinicians need to use clinical judgment.”
Clara cell secretory protein-16 (CC-16): acts as an immunosuppressant that provides protection against oxidative stress and carcinogenesis. Patients with COPD have reduced serum levels of CC-16 (Thorax 2008;63:1032-1034,1058-1063)…..”CC-16 cannot be used to screen populations of individuals for COPD and cannot be used as a surrogate for lung function testing to evaluate the presence of reversible airflow obstruction,” the investigators conclude. However, “serum CC-16 may be useful in longitudinal studies to assess epithelial repair or may be combined with other biomarkers to diagnose or monitor the progression of COPD.”….”its usefulness will be limited unless the measured levels of CC-16 can be shown to reflect the presence and severity of airway dysfunction, normalcy, or injury to Clara cells and changes in these states with reasonable speed. To date, this has not been done.”
Other: Elevated titers of circulating autoantibodies present in some patients with COPD suggest that it may have an autoimmune component (Am J Respir Crit Care Med. Posted online November 19, 2010)…..34% of patients had an abnormally high (= 1:160) ANA titer, vs. 3% of controls (p < 0.01). Less than 1% were positive for anti-dsDNA (double-stranded DNA) or anti-ENA (extractable nuclear antigen)…..ANA titers weren’t related to disease severity…..AT titers were similarly elevated in 26% of patients and about 6% of controls (p < 0.01). The authors tested for mitochondrial, liver-kidney microsomal, smooth muscle (SMA), and parietal gastric cell autoantibodies; more than 90% of AT-positive patients were SMA-positive.
pir Crit Care Med. Posted online November 19, 2010
Tx of COPD:
Links: Routine (Diet, Guidelines, Beta-Blockers) | Rehab | Tx: exacerbations | Meds | Step-Care Stages | Surgical | O2 | Chronic Bronchitis |
COPD is progressive and lung function can be expected to decline even with the best of care. Recommendations by the American College of Chest Physicians (Chest 2001;119:1185): CXR at admission may be useful, spirometry not useful to asses severity or dx exacerbation, inhaled bronchodilators (anticholinergic & short-acting B-2 agonists) beneficial, systemic corticosteroids X10-14 days if mod-severe exacerbation, narrow-spectrum Abx (Amox, TMP-SMX or TCN) preferable to broad-spectrum, mucolytics & methylxanthines not useful, there is no reliable method to risk stratify for relapse.
• If only have mild-mod obstructive lung dz, most pt’s will tolerate cardio-selective beta-blockers (if cardiac dz warrants) w/o incr sx’s or decr FEV1 (Cochrane Database 2001;3:CD002992).
• Megestrol Acetate 800mg PO qd may not only stimulate wt gain in underweight COPD pt, but improve ventilation per ABG’s (Chest 2002;121:1070).
Routine Outpatient Management: Smoking cessation to slow the accelerated decline in FEV1 (60ml/yr vs normal 30ml/yr) as no meds can prevent the decline of lung function in pt’s who smoke. Circadian rhythm- 4AM has highest airway resistance so use long acting Albuterol (Salmeterol) in PM, 3pm dose of steroids better than am dose in COPD. Bronchodilators, vaccinate for pneumococcus and flu. Need to address psychosocial issues and educate the pt individually and the family to motivate, but give realistic goal to restore pt to the highest level of independent function.
• Self management (prompt initiation of oral steroids & Abx x10d if 2 of 3 sx’s: dyspnea, sputum or sputum purulence) with exercise 3x/wk and other lifestyle modifications significantly reduces healthcare utilization and improves health status (Arch Int Med 2003;163:585-91).
• Combined therapy with two long-acting bronchodilators (formoterol qd-BID + tiotropium are better than tiotropium alone) confers a short-term improvement in pulmonary function in pt’s with COPD (Chest 2006 Mar; 129:509-17).
Diet: A diet high in fruit fiber (particularly apples, pears and grapes) and soy products may reduce the incidence of respiratory sx’s such as productive cough in COPD (Am J Respir Crit Care Med 2004;170:279-287), flavonoids may protect the lung on the basis of their antioxidant and anti-inflammatory properties.
• Nutritional support, mainly in the form of oral nutritional supplements, has a significant upside in patients with COPD, according to a new systematic review and meta-analysis (Am J Clin Nutr 2012;April 18th online)…..leads to “significant improvements in energy and protein intakes resulting in substantial increases in body weight by a level (~2 kg) that is associated with functional improvements.”
• Pt’s with COPD who take creatine supplements gain fat-free mass, increased peripheral muscle strength and endurance, and improved health status, however, whole body exercise capacity is not improved (Thorax 2005;60:531-537) (creatine monohydrate 5.7 g, glucose 35 g TID x 2wks, then qd x 10wk as took part in outpatient pulmonary rehabilitation).
• Individuals whose diets are rich in meat, refined starches and sodium are 1.43 times more likely to report persistent coughs with phlegm than those who consume a diet high in fruit and soy (Am J Resp Crit Care Med 2005;Nov).
• Omega-3 fatty acid supplementation improved lung function in pt’s with COPD (Chest. 2005;128:3817-3827).
• An antioxidant found in broccoli may reduce oxidative stress that leads to lung damage in COPD in mouse and cell line models (Am J Respir Crit Care Med 2008;178:552-54)…..Currently available glutathione-based antioxidants have proven disappointing against progression and exacerbations of COPD. Dietary counseling and food fortification can improve symptoms and general health status for patients with COPD who are at risk of malnutrition (Thorax 2009;64:326-331). A diet rich in raw fresh fruits, fruit juices, and vegetables may improve lung function and reduce exacerbations in patients with COPD according to a prospective randomized trial (Eur Respir J. 2010;online February 11).
• Excessive consumption of cured meat is associated with a 2-fold increased risk for hospital readmission among 274 patients with COPD (Eur Respir J. Published online March 8, 2012)…..Cured meat consumption was defined as the total daily consumption (g/day) of cooked ham, Spanish cured ham, cured and other sausages, and hot dogs…..the median cured meat intake was 23 g/day (equivalent to approximately 1 slice of ham a day).
Avoid: Avoid regular use of antitussives. Avoid indoor and outdoor air pollution (monitor public announcements regarding outdoor pollution and stay indoor during pollution episodes, avoid biomass fuels burned for cooking and heating in poorly ventelated areas). Anemia appears to be relatively common in pt’s with COPD (Chest 2005;127:825-829) (may be due to erythropoietin resistance), iron supplementation may improve exercise tolerance. Minimize acetaminophen use as may reduce levels of the antioxidant glutathione leading to increased risk of asthma and COPD (Am J Resp Crit Care Med 2005;171:966-71).
Beta-Blockers: Beta-blockers actually seem to decrease COPD exacerbations and improve survival…possibly by treating co-existing heart disease (Prescriber’s Letter. 2013;20:8)….Continue to use a CARDIOselective beta-blocker (bisoprolol, metoprolol, etc)…and avoid nonselective ones (carvedilol, etc). Start with a low dose and monitor lung function.
• In 20 studies of cardioselective beta blockers in pt’s with COPD, participants had no adverse pulmonary or respiratory effects. Because of their salutary cardiovascular effects, cardioselective beta blockers should not be withheld from pt’s with COPD (Cochrane Database Syst Rev 2005;(4):CD003566).
• Propranolol > Metoprolol impairs pulmonary function in pt’s with COPD, this effect should be weighed against the cardiovascular benefit of these agents (Chest 2005;127:818-824). Celiprolol, which is also cardioselective, has certain other adrenergic selectivities, and it did not significantly alter FEV1 or AHR compared with placebo, the investigators report.
• Beta blockers can be safely used in patients hospitalized with acute exacerbations of COPD according to a stduy with 825 hospitalized pt’s (Thorax 2008;63:301-305)…..The use of beta blockers and short-acting beta agonists was associated with reduced in-hospital mortality, as was an increase in the number of daily doses of beta blockers.
• Cardioselective beta-blockers appear to be safe and may improve outcomes for COPD patients undergoing vascular surgery (“Beta-Blockers Are Safe in Patients with Chronic Obstructive Pulmonary Disease, But Only with Caution” Am J Respir Crit Care Med 2008;178;661-666). …..63% reduction for COPD patients at 30 days (OR 0.37) vs 66% reduction for patients without COPD at 30 days (OR 0.34)….27% reduction for COPD patients over the entire follow-up period (HR 0.73) vs 16% reduction for non-COPD patients over the long term, although not significant (HR 0.84).
• Beta-blockers, long withheld from patients with chronic obstructive pulmonary disease over concerns that they would worsen symptoms, actually seem to lessen mortality and exacerbations according to a 7 year study on 2200 patients (average age, 65) (Arch Intern Med. 2010;170(10):880-887)……..The crude and adjusted hazard ratios with Cox regression analysis of beta-blocker use for mortality were 0.70 (95% CI, 0.59-0.84) and 0.68 (95% CI, 0.56-0.83), respectively……Subgroup analyses revealed that patients with COPD but without overt cardiovascular disease had similar results…..These data might not prompt us to prescribe beta-blockers to all COPD patients yet, but we can gain comfort in knowing that patients with COPD who possess other indications for Beta-blocker therapy are likely to attain substantial benefit.
• Four-year mortality was lower among 6000 Scottish patients (mean age at diagnosis, 69) with COPD who received beta-blockers (BMJ 2011;342:d2549)……819 patients used beta-blockers…..After a mean follow-up of 4.4 years (during which one third of patients died), beta-blocker use was associated with 22% lower all-cause mortality regardless of patients’ concurrent inhaled therapies (i.e., steroid, long-acting beta-2-agonist, anticholinergic, or combination therapy)…….had no adverse effects on lung function.
COPD Clinical Practice Guidelines: (Ann Int Med 2007;147:633-638)
The guideline recommends (strongly):
1. Spirometry to diagnose airflow obstruction for pt’s with respiratory symptoms, particularly dyspnea, but not for asymptomatic people.
2. Reserve tx for symptomatic pt’s with FEV1 <60% predicted. Tx should be monotherapy with a long-acting beta-agonist, long-acting inhaled anticholinergic, or inhaled corticosteroid. Although monotherapy reduces exacerbations, it does not reduce mortality rates.
3. Oxygen therapy for resting hypoxemia (Pa02 <55 mm Hg). O2 used for at least 15 hours daily, reduces mortality in pt’s with resting hypoxemia and FEV1 under 30% predicted.
Weak recommendations include:
1. Consideration of a combination of inhaled therapies for those with symptoms and FEV1 <60%.
2. Limiting use of pulmonary rehab to symptomatic pt’s with FEV1 under 50% predicted.
2013 GOLD Guildelines:
Grade patients (A-D) based on symptoms, airflow obstruction, and exacerbation history as follows:Previous versions of the GOLD guidelines classified patients by FEV1 only: mild/GOLD 1 (>=80%), moderate/GOLD 2 (50-79%), severe/GOLD 3 (30-49%), and very severe/GOLD 4 (<30%).
Symptom burden is measured by your choice of themodified Medical Research Council questionnaire (mMRC)or the COPD assessment test (CAT).
A = Low risk, low symptom burden:
First Choice: Rx Short acting beta agonist (SAMA) such as albuterol or short acting muscurinic antagonist (SABA) such as Respimat. Theophyline can be used at any of these stages.
Second Choice: Long-acting muscurinic antagonist (LAMA) or Long-acting beta agonist (LABA). Or a SABA + SAMA.
Low symptom burden (mMRCof 0-1 OR CAT score < 10) AND
FEV1 of 50% or greater (old GOLD 1-2) AND low exacerbation rate (0-1/year)
B = Low risk, higher symptom burden:
First Choice: LAMA or LABA. Along with a SABA or SAMA PRN.
Second Choice: LAMA + LABA
Higher symptom burden (mMRC of 2 or more OR CAT of 10 or more) AND
FEV1 of 50% or greater (old GOLD 1-2) AND low exacerbation rate (0-1/year)
C = High risk, low symptom burden:
Low symptom burden (mMRCof 0-1 OR CAT score < 10) AND
FEV1 < 50% (old GOLD 3-4) AND/OR high exacerbation rate (2 or more/year)
First Choice: LAMA or LABA + ICS. Along with a SABA or SAMA PRN.
Second Choice: Combo of two: LAMA, LABA or PDE4 inhibitor (Daliresp = Roflumilast)
D = High risk, higher symptom burden:
First Choice: LAMA or LABA + ICS + LAMA. Along with a SABA or SAMA PRN.
Second Choice: Combo of: LAMA, LABA, ICS or PDE4 inhibitor. Carbocysteine.
Higher symptom burden (mMRC of 2 or more OR CAT of 10 or more) AND
FEV1 < 50% (old GOLD 3-4) AND/OR high exacerbation rate (2 or more/year)
Statins: Statin use, particularly in conjunction with inhaled corticosteroid therapy, appears to improve survival following a COPD flare-up (Eur Respir J 2007;29:279-283). In patients with COPD, treatment with a statin may have a beneficial effect on several clinically relevant adverse outcomes, including COPD exacerbations and need for intubation (OR 0.43 and OR 0.1, respectively) and death from COPD (OR 0.19-0.29) or all-cause mortality (death from any cause, odds ratio/hazard ratio 0.48-0.67) according to a systematic review (BMC Pulm Med. 2009;9:32)…….statins also attenuated decline of pulmonary function…..The one randomized controlled trial investigated the effects of pravastatin 40 mg/day versus placebo over 6 months in 125 clinically stable COPD patients. In this trial, it was reported that statin users experienced significant improvements in exercise capacity and dyspnea after exercise in association with decreased levels of the inflammatory markers, C-reactive protein and interleukin-6, but no improvement in lung function…..”While statins seem to influence systemic inflammation and cardiovascular morbidity in COPD patients, it appears likely that they also directly target airway inflammation,” the reviewers note.
Other: Pt’s with frequent COPD exacerbations had a risk of death 4.3 times > that of pt’s experiencing no acute exacerbations requiring admission in a 5-yr period (Thorax 2005;60:925-931)….pt’s with up to 2 exacerbations faced a 2.2-fold increased risk of death. Physicians tend to underestimate the survival probability of pt’s hospitalized for acute exacerbations of COPD or asthma, and as a result, many pt’s may be inappropriately denied admission to intensive care units for intubation (BMJ Online First 2007;Nov)….The actual survival rate was 62% at the 180-day end point, whereas clinicians predicted a mean survival rate of 49%. The evidence (from more than 100 randomized, controlled trials) suggests that history and physical examination are not good predictors of airflow obstruction severity and that people with airflow obstruction benefit from treatment, primarily by experiencing fewer exacerbations. Long-acting treatments are better than short-acting treatments for reducing exacerbations, and oxygen reduces deaths among those with severe hypoxemia.
Use spirometry to diagnose — but not to screen asymptomatic pt’s for — airflow obstruction.
COPD/ Pulmonary Rehab: Alleviate the pt’s fear by giving them a basic understanding of the anatomy and functioning of the respiratory tract. Explain that the sense of suffocation can be controlled by techniques to promote breathing control. Will increase function and decrease hospitalization, but no change in survival. Outpatient pulmonary rehabilitation for 3 years can slow the progression of COPD (can reduce the decline in FEV1) and improve physical performance, according to study findings (BMC Pulmonary Medicine 2009;9;Jun 22). Physical-training programs (treadmill walking) can significantly increase the exercise capacity of pt’s with even far-advanced cases. These resistive and endurance exercises in addition to improving general physical conditioning, may strengthening the inspiratory / respiratory muscle strength and endurance and thereby improve exercise capacity. Patients with COPD should be referred earlier for pulmonary rehabilitation so that they can realize the benefits that the therapy offers (Am J Respir Crit Care Med. 2008;177(suppl):A556).
The American College of Chest Physicians (ACCP) Guidelines (Chest 2007;131:1S-3S): Recc a focus on strength training, especially in the upper extremities as an important component of pulmonary rehabilitation….in addition to the usual recommendations for aerobic exercise, such as walking. Both low- and high-intensity exercises produce positive clinical changes. hey do not yet recommended is respiratory muscle training even though there are some studies that show benefit, but the literature does not yet support it. Supplemental oxygen “may improve gains in exercise endurance,” particularly useful for the pt with severe exercise-induced hypoxia. The emphasis is on increasing independence, level of function, ability to perform ADL’s and improving quality of life. Best to start pulmonary rehab (x 6=12 weeks) immediately after discharge from the hospital, before the pt has fully recovered. Also useful in other lung diseases, such as pulmonary fibrosis, asthma and lung cancer.
Exercises: Avoid the downward spiral of dyspnea with deconditioning aggravating dyspnea causing the pt to mistakedly adjust by reducing activity further. Emphasizing leg strengthening training, then arm. Interval exercise (alternated 20 sec at 50% of short-term max exercise capacity with 40 sec at 10% of short-term max) is as effective as continuous, high-intensity exercise (>70% of maximal exercise capacity) for improving short-term quality of life and exercise capacity in pt’s with severe COPD; however, interval exercise was better tolerated (Ann Intern Med 2006;145:816-25) (all performed aerobic exercise at home for at least 20 minutes per day).
• Pulmonary rehabilitation produces significant benefits for pt’s with advanced emphysema and plays an important role in the selection of pt’s for possible surgery (CHEST 2005;128:3783-3784,3799-3809) (some found to be too ill or fragile for surgery).
• COPD pt’s have low levels of physical activity during and after hospitalization for an acute exacerbation, thus enhanced physical activity should be included in the management of these pt’s (Chest 2006;129:536-544)…start by more walking and standing as often fearful of becoming breathless and therefore try to limit activity.
• A home-based respiratory muscle endurance training routine using tube breathing led to a significant improvement in exercise endurance capacity and other benefits in pt’s with COPD (Chest 2006;129:886-892) (tube connected to a mouthpiece increases dead space and prompts rebreathing of exhaled carbon dioxide, 15 minutes qd).
• High intensity inspiratory muscle training can improve muscle function in COPD pt’s and reduce dyspnea and fatigue (Eur Respir J 2006;27:1119-1128), if used alone, such training is unlikely to yield clinically relevant improvements in exercise capacity.
• Neuromuscular electrostimulation (ES) is better than usual rehabilitation in improving strength and dyspnea in severely deconditioned and malnourished pt’s with COPD and low BMI (Chest. 2006;129:1540-1548).
• The ES program consisted of 4 days per week of electrically induced contractions of the quadriceps performed 4 times a week using an electrostimulator with 3 skin-surface electrodes for each quadriceps for 30 minutes on both legs simultaneously. Intensity was maximal tolerated by the pt and increased by 1 to 5 mA daily. COPD pt’s who engage in regular physical activity, even a relatively small amount (light physical activity, such as biking or walking, for <2 hours/week), have a decreased (28% less) risk of hospital admission and death (Thorax. 2006;61:772-778).
• Moderate to high levels of physical activity are linked with reduced lung function decline and risk for COPD (and exacerbation) among smokers (Am J Respir Crit Care Med. 2007;175:458-463).
• Testosterone supplementation in men with COPD and low testosterone levels improves lean body mass and strength without significant adverse effects (Am J Resp Crit Care Med. 2004;170:870-878).
• Training the expiratory muscles can lead to improvement in both strength and endurance and increase exercise performance (Chest 2003;124:468-473), also improves the cough and reduces the sensation of respiratory effort during exercise. Exercising one leg at a time can improve aerobic capacity more than two-legged exercise in patients with stable COPD (Chest 2008;133:337-339,370-376)…..”One-legged exercise, at half the load of two-legged exercise, places the same metabolic demands on the targeted muscles but reduces the ventilatory load, enabling patients to increase work capacity.”….easy to do, inexpensive, and it’s simple to modify a stationary bike….The best patients are clinically stable with severe lung disease who would otherwise be too short of breath on minimal exertion to undertake any meaningful exercise training.” Distracting auditory stimuli delivered via headphones helps reduce the unpleasantness of dyspnea in COPD patients, and such interventions might be an aid to exercise adherence (Chest 2007;132:1506-1512). Patients with moderate COPD can maintain the improvements they obtain from an 8-week pulmonary-rehabilitation program for at least 1 year provided that they continue to exercise regularly (European Respiratory Society (ERS) 19th Annual Congress: Abstract 4640. Presented September 16, 2009).
• The benefits of exercises to strengthen muscles in the upper arms, shoulders, and chest of patients with COPD extend beyond the upper extremities a clinical trial on 25 pts (mean age 69 years) indicates (Chest. 2009;136:387-395)…..provides data regarding the benefits of this training on clinically important outcomes, such as the ability to perform activities of daily living that involve the upper extremities, general exercise capacity and the fatigue related to those activities.
• Tai Chi (combines movement with deep breathing) can serve as an effective means of exercise training in patients with COPD according to research from Australia (Eur Respir J 2012;August 9th online)…..”The improvement in balance, together with the significant improvement in lower limb quadriceps strength after Tai Chi training, shows that 12 weeks of the short-form Sun-style Tai Chi has the potential to reduce falls in people with COPD.” ….Perceived exertion during the endurance shuttle walk test was significantly less in the Tai Chi group than in the control group.
• Resistance arm training has no effect on dyspnea or health-related quality of life in patients with COPD according to a study on 36 pt’s (mean age 66) (Chest. 2011;139:151-158)…..There were no between-group differences in dyspnea during activities of daily living, health-related quality of life, or symptoms during exercise….However, the active intervention group showed significant improvements on the 6-minute pegboard and ring test (p=0.03) and in unsupported arm exercise capacity (p=0.01).2
Exertional dyspnea: Combined tx using bronchodilators and oxygen can significantly improve exertional dyspnea in pt’s with normoxic COPD (Thorax 2006;61:559-567). Exercise endurance time increased by 1.7 minutes (16%) after bronchodilator therapy, by 3.1 minutes (28%) with oxygen therapy, and by 5 minutes (40%) with the combination.
Controlled breathing techniques (diaphragmatic –> 20 min TID –> change breathing pattern from using ribs as primary pressure generators to using diaphragm (taught as pt supine and hand on abd, should move up on insp, then exhale through pursed lips) forward bending and pursed-lip breathing –> inhale through nose and exhale through lips pursed in a whistling of kissing position), chest PT/ postural drainage to enhance removal of secretions. –> less dyspnea, anxiety, panic attacks and improved sense of well being.
• A one year self-management program for COPD pt’s did not reduce adverse outcomes (BMJ 2012;344:e1060)….the self-management program, which included four training sessions every 2 weeks followed by nurse home visits every 6 weeks….The goal was to enable participants to understand their disease, monitor symptoms and activate a self-management plan including adjusting treatment early in the course of an exacerbation (e.g., adding an oral antibiotic and/or corticosteroid) and initiating contact with a medical provider…..there was no difference in hospital readmission or death due to COPD between the intervention group and the control group (48% and 47%).
American College of Chest Physicians (ACCP) and American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) Guidelines for pulmonary rehabilitation: (Chest. 2007;131:4S-42S)
Specific recommendations in the updated guidelines are as follows:
• For patients with COPD, a program of exercise training of the muscles of ambulation should be a mandatory component of pulmonary rehabilitation (grade of recommendation, 1A).
• In patients with COPD, pulmonary rehabilitation improves the symptom of dyspnea (1A).
• In patients with COPD, pulmonary rehabilitation improves health-related quality of life (1A).
• In patients with COPD, pulmonary rehabilitation reduces the number of hospital days and other measures of healthcare utilization (2B).
• In patients with COPD, pulmonary rehabilitation is cost-effective (2C).
• Evidence is insufficient to determine if pulmonary rehabilitation improves survival in patients with COPD.
• Comprehensive pulmonary rehabilitation programs provide psychosocial benefits to patients with COPD (2B).
• Although 6 to 12 weeks of pulmonary rehabilitation are associated with benefits in several outcomes, these decline gradually during 12 to 18 months (1A). However, some benefits, including health-related quality of life, are maintained above control levels at 12 to 18 months (1C).
• Longer pulmonary rehabilitation programs (12 weeks) are associated with greater sustained benefits than are shorter programs (2C).
• Maintenance strategies after pulmonary rehabilitation are associated with a modest improvement in long-term outcomes (2C).
• Compared with lower extremity exercise training at lower intensity, higher exercise intensity is associated with greater physiologic improvement in patients with COPD (1B).
• For patients with COPD, both low- and high-intensity exercise training produce clinical benefits (1A).
• Adding a strength training component to a program of pulmonary rehabilitation increases both muscle strength and muscle mass (1A).
• Routine use of anabolic agents in pulmonary rehabilitation for patients with COPD is not supported by current scientific evidence (2C).
• In patients with COPD, unsupported endurance training of the upper extremities is beneficial and should be included in pulmonary rehabilitation programs (1A).
• Routine use of inspiratory muscle training as an essential component of pulmonary rehabilitation is not supported by currently available scientific evidence (1B).
• Education on collaborative self-management and prevention and treatment of exacerbations should be an integral component of pulmonary rehabilitation (1B).
• Evidence to support the benefits of psychosocial interventions as a single therapeutic modality is minimal (2C).
• Current practice and expert opinion support including psychosocial interventions as a component of comprehensive pulmonary rehabilitation programs for patients with COPD. However, scientific evidence is lacking, and therefore no recommendation is provided.
• In patients with severe exercise-induced hypoxemia, supplemental oxygen should be used during rehabilitative exercise training (1C).
• In patients without exercise-induced hypoxemia, administering supplemental oxygen during high-intensity exercise programs may improve gains in exercise endurance (2C).
• In selected patients with severe COPD, noninvasive ventilation as an adjunct to exercise training produces modest additional improvements in exercise performance (2B).
• Evidence is insufficient to support the routine use of nutritional supplementation in pulmonary rehabilitation of patients with COPD.
• For some patients with chronic respiratory tract diseases other than COPD, pulmonary rehabilitation is beneficial (1B).
• Current practice and expert opinion suggest that pulmonary rehabilitation for patients with chronic respiratory tract diseases other than COPD should be modified to include treatment strategies specific to individual diseases and patients, as well as treatment strategies common to both COPD and non-COPD patients. However, scientific evidence to support this recommendation is lacking.
Tx of Exacerbations: Topics: Pearls | Corticosteroids | Bronchodilators | Abx | O2 | Heliox | Hospitalization, ICU Criteria & Ddx |
COPD exacerbation is “an event in the natural course of the disease characterized by a change in the pt’s baseline dyspnea, cough, and/or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication in a pt with underlying COPD” (GOLD Executive Summary. Am J Respir Crit Care Med 2007;176:532-555)…..Abx’s are recommended for pt’s with the following exacerbations: Increased dyspnea, sputum volume, and sputum purulence (Evidence B). Increased purulence of sputum and either increased dyspnea or increased sputum volume (Evidence C). A severe exacerbation requiring mechanical ventilation whether invasive or noninvasive (Evidence B).
Pearls: pOx often misleading as can have severe metabolic derangements in pH & PCO2. (always get ABG if pt has FEV1 <35% predicted).
Most are due to viral infections, but often due to bacteria (typical and atypical), amount & type of sputum may be misleading, G-stain & Cx not routinely indicated due to the 48-72hr delay in Cx results. Strongly consider a CXR as it may demonstrate an abnormality that may change your management in 10%. Be wary of discharging pt’s with exacerbations when they do not feel comfortable with their breathing, regardless of their oxygen saturation, ABG, or other test results. Always look for underlying cardiac ischemia with acute exacerbations. With hypoxia and distress, many of these pt’s can have unrecognized underlying ischemia.
• Self-management of COPD (monitor symptoms including adding an oral antibiotic and/or corticosteroid and initiating contact with a medical provider) did not reduce adverse outcomes according to a Scottish trial, which involved 464 patients hospitalized for acute COPD exacerbations (BMJ 2012;344:e1060)……successful self-managers, who were younger and more likely to be living with others than unsuccessful self-managers.
Corticosteroids: Helpful if chronic bronchitis (not very helpful if emphysema).
• A 5-day course of glucocorticoids (prednisone 40 mg/day) is sufficient to treat acute exacerbations of COPD, according to a placebo-controlled noninferiority trial (5 vs 14 days) on 300 pt’s presenting to the ED (JAMA 2013;309:2223-31)…..Editorialists write: “The clinical implications of this study are clear. Most patients with acute COPD exacerbations can be treated with a 5-day course of prednisone or equivalent.” Higher doses cause more adverse effects…and IV steroids are not more effective (Prescriber’s Letter. 2013;20:8).
• Methylprednisolone (Solumedrol) 60-125mg IV q6-8h X 3d, then PO if initially unable to tolerate PO’s.
• Short courses of systemic steroids in acute COPD exacerbations improves both clinical and spirometric outcomes (Arch Int Med 2002 162:2527-36).
• If take >1,000 mg prednisolone on an intermittent basis, then high risk for osteoporosis (Chest 2002;121:1456).
• Oral prednisone therapy (40mg/d x10d with TMP-SMX or 100mg Doxy BID) can reduce the risk of relapse in pt’s with exacerbation of COPD, side effects include increased appetite, wt gain and insomnia (NEJM 2003;348:26:2618-25).
• Early and aggressive tx of COPD exacerbations results in improved recovery (Am J Respir Crit Care Med 2004;169:1267-1268, 1298-1303), oral corticosteroids gave a tx recovery time 2.63 days shorter compared with exacerbations not treated with oral prednisolone.
• When pt’s with COPD are treated with an inhaled corticosteroid, they are less likely to experience ischemic cardiac events (European Respiratory Society. Abstract 2333. Presented Sept. 19, 2005).
• COPD pt’s who inhaled corticosteroids had a 70% increase in the risk of pneumonia hospitalization over those not given the drugs according to a nested case-control study (Am J Respir Crit Care 2007;176:162-166).
• A RCT found no advantage for IV vs PO (60 mg daily x 5, then taper) steroids for COPD exacerbations hospitalized in non-ICU settings (Chest 2007;132:1741)….not surprising, given the high bioavailability of prednisone and prednisolone after oral administration…..Given its convenience, cost, and the finding that it is not inferior to intravenous prednisolone, “we therefore suggest that oral prednisolone therapy is the preferred route of administration for most patients admitted to the hospital with an exacerbation of COPD,” the authors conclude.
• In patients hospitalized for exacerbations of acute COPD, low-dose corticosteroids (30 to 40 mg/d) given orally were not associated with worse outcomes than those seen with high-dose intravenous corticosteroids, according to the results of a large, observational, pharmacoepidemiological cohort study (JAMA. 2010;303:2359-2367, 2409-2410).
Bronchodilation: Meds | Ipratropium (Atrovent) & Albuterol NEBS/ MDI. Incr beta agonist with spacer to q30 min-2h or SC epi or terbutaline 0.1-.5ml. Ipratropium alone or combined with short-acting B2 agonist does not increase the degree of bronchodilation (not additive) during acute exacerbations at 90min or 24hr (monotherapy may be equally effective) (Cochrane Database Syst Rev 2002;CD003900).
• For acute exacerbations of COPD, 2.5 mg q4hr of nebulized albuterol is as good as 5 mg (Chest. 2005;128:48-54) (same maximal bronchodilation, length of hospital stay and recovery).
Abx’s in COPD: Bacterial likely a causative role in exacerbation (NEJM 2002;34:465). Base on local pattern of Abx sensitivities. Bacteria colonize the airways of adults with COPD and release myriad potent inflammatory molecules (endotoxin, peptidoglycan fragments, outer membrane lipoproteins, lipoteichoic acid, microbial toxins etc), ~50% of exacerbations are caused by bacteria (Curr Opin Inf Dis 2006;19:225–230) (particularly non-encapsulated H. influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae).
5-10-day course of a narrow spectrum Abx if early stage COPD:
TMP-SMX BID.
Doxy 100mg BID.
Amox 500mg TID.
If risk factors (severe COPD, frequent exacerbations, coexisting cardiac dz) use:
Amox-Clav 500-875mg BID
Quinolone: Levaquin etc.
Zithromax (500mg PO qd X3 approved by FDA, most recommend 5 days). 5 day Z-pak similar to 7 day Levofloxacin (Chest 2003;123:772-7).
Biaxin 500mg BID. • More experts now recommend not using azithromycin or other macrolides for most acute respiratory infections as pneumococcal infections are becoming more resistant….use a beta-lactam (penicillin, amoxicillin, etc) (Prescriber’s Letter. 2013;20:8).
Cefuroxime.
Moxifloxacin was as effective as amoxicillin/clavulanic acid for COPD exacerbations in outpatients in the MAESTRAL trial (Eur Resp J 2011;online December 1).
Info: In patients with acute exacerbations of chronic bronchitis and COPD, a short course of antibiotics (5 days or less) is as effective as a standard longer course according to a double-blind study (Thorax 2008;63:415-422).
• For 170 patients with severe acute exacerbation of COPD, trimethoprim-sulfamethoxazole (160/800 mg BID) was just as effective and safe as ciprofloxacin (750 mg BID) in a double-blind prospective trial (Clin Infect Dis 2010;51)…..The combined rate of hospital deaths and the need for additional antibiotic courses — the primary outcome — was 16.4% in the trimethoprim-sulfamethoxazole group and 15.3% in the ciprofloxacin group. The mean exacerbation-free intervals were 83 and 79 days, respectively.
• A retrospective cohort study of nearly 85,000 patients admitted for COPD exacerbations found that pt’s treated with antibiotic therapy (most commonly with quinolones, cephalosporins, or macrolides) were significantly less likely than untreated patients to receive mechanical ventilation (1.1% vs. 1.8%), die (1.0% vs. 1.6%), or to be readmitted for COPD (7.9% vs. 8.8%)(JAMA 2010;303:2035)…..However, treated patients also had a higher rate of readmission for C difficile infections than untreated patients (0.2% vs. 0.1%)…….adjusted for a wide range of clinical and demographic factors, composite risk for treatment failure was 13% lower in the treated group…..antibiotic use appears to be appropriate for all hospitalized patients with COPD exacerbations.
Supplemental O2: temporarily incr by 0.5-1L/min. If not previously in, give @ ~2-4L/min with caution (aim for 90-92% sat), guided by P-ox/ ABG, use Venturi (controlled flow) mask if have CO2 retention and concomitant hypoxia as risk respiratory failure. Check room air ABG before d/c home to document O2 needs. PRN postural percussion and drainage (PP&D) or vibration therapy. O2 is the only tx that is proven to extend life, give if SATs <55mmHg in usual state of health or <60 and evidence of chronic hypoxemia (polycythemia, ankle edema, venous engorgement, EKG with p-pulmonale, psych impairment). Inhaled steroids of no benefit except in the small group that has reversible bronchospasm. Hydration to keep urine clear.
Heliox (low density, low viscosity reduces hypoventilation/ fatigue), reduces risk of intubation and mortality (Crit Care Med 2001;29:2322). Heliox usually is in a 60:40 mixture of helium with oxygen. Helium is a smaller particle than oxygen and in small airways promotes laminar flow and facilitates both oxygen transport and carbon dioxide diffusion. Many pt’s who seem to breathe better on Heliox return to a worsened respiratory state when removed from Heliox.
• Inhaling a combination of 72% helium and 28% O2 during exertion increases the comfortable walking distance and reduces the perception of exertional difficulty in pt’s with stable COPD (Am J Respir Crit Care Med 2006;173:865-70).
• Compared to use of oxygen, breathing a helium-hyperoxia mixture (70% helium and 30% oxygen via a mask) increased exercise tolerance (walking distance) considerably in pt’s with COPD (Chest 2007;131:1659-1665).
Nocturnal noninvasive nasal ventilation appears to extend the lifespan of patients with stable hypercapnic COPD, but the approach is not without disadvantages (Thorax 2009;64:553-556,561-566)…..showed small but significant increases in REM sleep and decreases in the frequency of disordered breathing events and a sleep-related rise in transcutaneous PCO2…..also shown to produce an improvement in survival (adjusted hazard ratio, 0.63), but no changes in daytime arterial blood gas, lung function measurements, or hospitalization rates.
Management of COPD exacerbations:
(Am Fam Physician. 2010;81:607-613)
* Noninvasive positive pressure ventilation improves respiratory acidosis while reducing respiratory rate, breathlessness, need for intubation, mortality rate, and length of hospital stay (level of evidence, A).
* In patients with COPD, inhaled bronchodilators (beta-agonists, alone or in combination with anticholinergic agents) reduce dyspnea and improve exercise tolerance (level of evidence, A).
* In patients with COPD, short courses of systemic corticosteroids prolong the time to subsequent exacerbation, reduce the rate of treatment failure, reduce length of hospitalization, and improve forced expiratory volume in 1 second and hypoxemia (level of evidence, A).
* Compared with high-dosage corticosteroid regimens, low-dosage regimens are not inferior in reducing the risk for treatment failure in patients with COPD (level of evidence, B).
* Compared with intravenous prednisolone, oral prednisolone is equivalent in lowering the risk for treatment failure in patients with COPD (level of evidence, B).
* Oral corticosteroids are bioavailable, inexpensive, and convenient, and are therefore recommended in patients who can safely swallow and absorb them (level of evidence, B).
* Patients with moderate or severe COPD exacerbations should be treated with antibiotics, especially in patients with increased sputum purulence or who need to be hospitalized (level of evidence, B).
* Symptoms, such as presence of purulent sputum; recent use of antibiotics; and local patterns of microbial resistance should help determine the choice of antibiotic in patients with COPD (level of evidence, C). Evidence is limited that broad-spectrum antibiotics are more effective than narrow-spectrum antibiotics.
* In patients with COPD, smoking cessation lowers the mortality rate and likelihood of subsequent exacerbations (level of evidence, A).
* In severely ill patients with COPD, long-term oxygen therapy reduces the risk for hospitalization and duration of hospitalization (level of evidence, B).
* To qualify for discharge, a patient should have stable clinical symptoms and a stable or improving arterial partial pressure of oxygen of more than 60 mm Hg for at least 12 hours [and] not require albuterol more often than every four hours.
* If the patient is stable and can use a metered dose inhaler, there is no benefit to using nebulized bronchodilators.
Doxy: In a small randomized trial at two medical centers in the Netherlands (223 patients; 265 exacerbations), 30-day outcomes were no better with doxycycline (x 7 days) than with placebo in treating acute exacerbations of COPD (Am J Respir Crit Care Med 2010;181:150)……clinical success (cure or improvement) at day 30 — was observed in 78 (61%) of the exacerbations randomized to doxycycline compared with 72 (53%) of those randomized to placebo (P=0.32)……Per inclusion criteria, none of the patients had fever (temperature 38.5°C), recent antibiotic treatment for 24 hours, or radiographic evidence of pneumonia….. For each exacerbation, participants received a fixed tapering dose of intravenous and oral prednisolone as well as nebulized bronchodilator therapy…..there was evidence of short-time symptomatic improvement with doxycycline, manifested by fewer dropouts, less need for open-label antibiotics, and other improvements at day 10.
Hospitalize if: Criteria for inpatient management:
• Pulmonary sx’s unresponsive to outpatient tx.
• Severe underlying disease. Severe background COPD. New or worsening cor pulmonale.
• Significant comorbid illness contributing to the exacerbation of pulmonary sx’s.
• Older age (>70 years).
• Onset of new severe symptoms, such as dyspnea at rest or new signs, such a peripheral edema or cyanosis.
• Diagnostic uncertainty.
• Worsening hypercapnea or hypoxemia change in mental status.
• Poor home support. Pt’s unable to take care of themselves as outpt’s.
ICU if: persistent or worsening hypoxemia despite max supplemental O2. Severe or worsening respiratory acidosis (pH <7.30). Signs of impending respiratory failure (paradoxical breathing). Confusion or lethargy. Assisted ventilation (invasive or noninvasive) is required or planned.
R/o Pulmonary Embolism (PE): PE is common (25% per CT-angiography) in COPD exacerbation of unknown origin (no evidence of lower respiratory tract infection, pneumothorax or iatrogenic cause) requiring hospitalization and sx’s are similar (Ann Intern Med 2006;144:390-6) (prior VTE, a decrease in PaCO2 of 5 mm Hg from baseline and malignancy were significantly associated with PE).
• Pt’s admitted for acute exacerbation of COPD appear to be at major risk of VTE (up to 10%) (Thromb Res 2003;112:203-207), implementation of prophylaxis with LMWH should be considered in all as the dx of concomitant PE may often be missed because sx’s of acute exacerbation of COPD mimic PE, and non-invasive evaluation by pulmonary scintigraphy or CT scan is less specific.
• A meta-analysis of study data (550 patients) suggests that up to one of every four patients hospitalized for an exacerbation of COPD has a pulmonary embolism (Chest 2009;135:786-793)…..By contrast, among patients discharged in the ER, the rate was just 3.3%.
Steroids: A retrospective cohort study, based on data from 414 U.S. hospitals with nearly 80,000 patients admitted for COPD to non–intensive care unit settings found that oral low-dose use (median, 60 mg for the first 2 days) was associated with less treatment failure than was high-dose parenteral use (equivalent to a median dose of 600 mg of prednisone total for the first 2 days) (JAMA 2010;303:2359)…..Treatment failure — defined as need for mechanical ventilation after the first 2 days, death, or readmission for COPD within 30 days — occurred in 11% of all patients……In analyses adjusted for about 50 clinical and demographic variables, as well as propensity scores, treatment failure was 16% lower in patients who received oral low-dose steroids than in those who received parenteral steroids; length of stay and cost were about 10% lower in the low-dose group……A worrisome secondary finding is that the vast majority of COPD patients received high-dose parenteral steroids, despite the contrary recommendations of major national and international guidelines — including those of the Global Initiative for Chronic Obstructive Lung Disease (GOLD).
Assisted ventilation as an alternative to intubation –> full face mask (secured with two straps for tight seal) connected to a mechanical ventilator with standard ventilator tubing for noninvasive positive pressure ventilation (NIPPV). BIPAP (I:E ration ~10:5. Insp pressure of 10, titrate to 12-15. Exp pressure 3-5). Most effective if hypercapnic failure with rising PaCO2 in the range of 55-75mmHg. (Lancet 1999;354). Lung volume reduction surgery (resect 1/3) for pink puffer to improve elastic recoil and raise diaphragm. (Acute exacerbations of COPD. NEJM 2002;346:13)
• In pt’s with severe COPD, long-term ambulatory use of pulsed nitric oxide (NO) inhaled with oxygen improves pulmonary hemodynamics (Thorax 2003;58:289-293,283-284), this may be due to the fact that endothelial cell release of nitric oxide appears to be impaired in hypoxic lung disease, suggesting that NO replacement could reduce the hypoxia-induced pulmonary HTN seen in severe COPD.
Invasive Ventilation: Patients should be considered for invasive ventilation if they meet one or more of the following criteria:
• Patients with severe dyspnea, use of accessary muscles and paradoxical abdominal motion
• Impending respiratory failure and life-threatening acid–base disturbances, i.e.: acidosis (pH < 7.25) hypercapnea (PaCO2) > 60 mmHg, 8.0 kPa.
• Respiratory frequency > 35 breaths per minute.
• Respiratory arrest.
• Impaired mental status, somnolence.
• NIPPV failure or contraindications.
• Cardiovascular complications, including hypotension, shock, or heart failure.
Ï Other complications, including metabolic abnormalities, sepsis, pneumonia, pulmonary embolism, barotrauma, and massive pleural effusion.
Ddx: pneumonia, CHF, myocardial ischemia, PTX, PE, URI, recurrent aspiration, systemic illness that increases ventilatory demand with underlying severe COPD (early sepsis).
Medications: Links: Info | Combo Tx | Steroids & Roflumilast | Mucolytics | Albuterol | Levalbuterol | Anticholinergics | Theophylline | O2 | Asthma MEDS | Nebulizer | Long-Acting Beta2 Agonists | Combo corticosteroid/beta-agonist | Tx of exacerbations | Dyspnea in End-Stage Pt |
No current COPD drug reduces mortality, but meds will reduce exacerbations and slow the decline in quality of life. Ipratropium and Tiotropium are first line (MDI form only). Don’t use inhaled steroids alone to treat COPD…..Consider them as add-on therapy to long-acting bronchodilators like salmeterol or tiotropium (Spiriva)…in severe COPD pt’s who have frequent exacerbations. Pt’s taking high-dose inhaled steroids get pneumonia more frequently….you only need to use Advair instead of Serevent in 16 pt’s for 3 years to cause one additional pneumonia. Inhaled therapy with fluticasone + salmeterol was associated with a 2.6% reduction in mortality compared to monotherapy (was not significant) (NEJM 2007;356:775-89) (All-cause mortality, the primary endpoint was 12.6% with combined therapy, 13.5% with salmeterol, 16% with fluticasone, and 15.2% with placebo)…. Average reduction of 0.12 exacerbations a year. Dry powder inhalers much easier to use in the elderly and should be first line before NEBs and other MDI’s. For Abx’s, see above.
• Results from the TORCH study suggest that inhaled therapies (either inhaled fluticasone alone, inhaled salmeterol alone, combined fluticasone/salmeterol but not placebo) can blunt lung-function decline by about 15 mL annually (Am J Respir Crit Care Med 2008;178:332)…..an editorialist comes down squarely in favor of monotherapy with the long-acting beta-agonist salmeterol: It avoids the potential long-term side effects of inhaled steroids, and its effects on mortality and hospitalizations (reported last year) were similar to those of combined steroid and beta-agonist therapy…..inhaled steroids reasonably can be added when needed to control symptoms in patients with advanced COPD.
• Treatment of COPD may be best started at an early stage according to RCT (Lancet. 2009;Published online August 28)…..”Tiotropium seemed to reduce the rate of decline of postbronchodilator FEV1 in patients with GOLD stage II COPD.”
Prophylactic Abx’s?: The addition of daily azithromycin 250 mg (n = 558) (antimicrobial and immunomodulary effects) vs placebo (n = 559) daily for 1 year to conventional treatment for COPD significantly helps reduce acute exacerbations associated with the disease (American Thoracic Society (ATS) 2011 International Conference: Abstract 22714. Presented May 17, 2011)…..the median time to the first exacerbation was 266 days in the azithromycin group and just 174 days in the placebo group…..The frequency of acute exacerbations between the 2 groups was also significantly different, with the azithromycin group experiencing 741 acute exacerbations, translating into 1.47 episodes per patient year, and the placebo group experiencing 900 exacerbations, or 1.84 episodes per patient year (P = .004).
Azithromycin: Adding azithromycin 250 mg/day to standard COPD therapy reduces the risk of acute exacerbations, but may worsen hearing (Prescriber’s Letter 2011;18:10)…..one less exacerbation for every 3 COPD patients on oxygen or with prior exacerbations that take azithromycin for one year…but one in 20 patients will experience slight hearing loss…..benefit is mostly due to azithromycin’s anti-inflammatory and immunomodulatory effects….Consider azithromycin for patients with severe COPD and frequent hospitalizations for acute exacerbations. Don’t use a macrolide for an acute exacerbation if the patient is on chronic azithromycin. Instead, use a quinolone, cephalosporin, or amoxicillin/clavulanate.
• The addition of daily azithromycin 250 mg (n = 558) (antimicrobial and immunomodulary effects) vs placebo (n = 559) daily for 1 year to conventional treatment for COPD significantly helps reduce acute exacerbations associated with the disease (American Thoracic Society (ATS) 2011 International Conference: Abstract 22714. Presented May 17, 2011)…..the median time to the first exacerbation was 266 days in the azithromycin group and just 174 days in the placebo group…..The frequency of acute exacerbations between the 2 groups was also significantly different, with the azithromycin group experiencing 741 acute exacerbations, translating into 1.47 episodes per patient year, and the placebo group experiencing 900 exacerbations, or 1.84 episodes per patient year (P = .004).
• A strategy being used to prevent exacerbations of COPD, with some success, is long-term antibiotic prophylaxis (American College of Clinical Pharmacy (ACCP) 2012 Annual Meeting, October 21 to 24)…..Approximately half of COPD exacerbations may be due to bacterial infection. COPD patients are commonly colonized with bacteria. Common offenders include Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarhallis, and Pseudomonas aeruginosa (which may be more important in advanced stages of COPD). The prophylactic role of oral macrolides (erythromycin, clarithromycin, azithromycin) and respiratory quinolones (mainly moxifloxacin) has been studied in COPD exacerbations. Oral therapies involving pulsed azithromycin 3 times per week have been studied over the course of a year in a small group of patients. Prophylactic inhalation therapies for COPD exacerbations require more study since the available data have evolved from cystic fibrosis inhalation trials. The prevalence of resistance to macrolides was 52% at study entrance. Of the 68% of patients [randomized to azithromycin] who were not colonized at enrollment, [81%] developed resistance during the study period. [Compare this with 70% of those randomized to placebo who were not colonized at the time of enrollment; only 41% of those participants developed resistance during the study period; P < .001.]
Combo Tx: One-year tx with tiotropium (Spiriva 18 µg once daily) + fluticasone & long-acting beta2-agonist (Advair 250/25 µg/puff, 2 puffs twice daily) improves lung function and quality of life in pt’s with moderate or severe COPD (Ann Intern Med. 2007;146:Feb) (did not statistically influence rates of exacerbations, 2% less) (40% discontinued tx prematurely). See Combo corticosteroid/beta-agonist |
• Tiotropium (18 mcg qd) used in combination with formoterol (12 mcg BID) was superior to the use of fluticasone (250 mcg BID) and salmeterol (25 mcg BID) in pt’s with moderate COPD (European Respiratory Society. Sept 18, 2005. Abstract 217). A 2-yr RCT 1323 patients with severe COPD with Tiotropium (18 µg qd) vs.
• Salmeterol/Fluticasone (50/500 µg BID) found that exacerbation rates were similar between groups; mortality rate was lower with salmeterol/fluticasone (3% vs. 6%, P=0.03)(Am J Respir Crit Care Med 2008;177:19).
• Combination therapy with tiotropium (qd) and formoterol (BID) achieves great improvement in daytime lung function (FEV1) in patients with moderate COPD than does salmeterol and fluticasone (both BID)(Chest 2008;134:255-262).
• Therapy with two long-acting bronchodilators might be a way to avoid long-term inhaled steroids in COPD patients who require more than one drug for symptom control (Chest 2008;134:255)…..In this randomized trial, researchers compared the spirometric effects of two combinations — tiotropium (Spiriva) plus formoterol and fluticasone plus salmeterol (a steroid plus a long-acting beta-agonist) — in 605 patients with moderate COPD…….overall 12-hour FEV1 response was also greater in the tiotropium-plus-formoterol group.
• Results from a study with 6000 patients with moderate-to-severe COPD suggest that inhaled therapies can blunt lung-function decline by about 15 mL annually (TORCH study. Am J Respir Crit Care Med 2008;178:332)….Compared with the rate of FEV1 decline in the placebo group (55 mL annually), the rate of decline was significantly lower in each of the three treatment groups (42, 42, and 39 mL annually in the fluticasone, salmeterol, and combined-therapy groups, respectively).
• In a controlled trial of pt’s with COPD using combined salmeterol/fluticasone inhalation therapy, withdrawal of fluticasone propionate led to an acute and persistent deterioration of lung function and dyspnea, and an increase in mild exacerbations of COPD and sleep disturbances (Thorax 2005;60:480-487).
• ICS therapy reduce all-cause mortality in COPD (Thorax 2005;60:992-997). Risk for moderate COPD exacerbations (lowered from 20.1% to 17.5%), but not severe exacerbations, was lower with combined therapy (long-acting bronchodilators with inhaled steroids) according to a systematic review (Chest 2009;136:1029)……Combination therapy was associated with significant excess risk for pneumonia (5.0% vs. 3.4%).
Triple Therapy: Mortality was lower with triple therapy (tiotropium + ICS + LABA) than with double therapy (ICS + LABA) according to a 4.7yr a retrospective cohort study of 1853 COPD patients (Chest 2012;141:81)……Although spirometric measures of COPD at baseline were worse in the triple-therapy group than in the double-therapy group, crude mortality was 37% lower in the triple-therapy group……In analyses that were adjusted for baseline differences between groups, triple therapy was associated with significantly lower all-cause, respiratory, and cardiovascular mortality than was double therapy……Rates of COPD exacerbations and hospital admissions for respiratory disease were also lower with triple therapy……This study of COPD patients doesn’t carry the weight of a randomized trial, and it doesn’t address day-to-day symptom control. Still, it boosts confidence that, for certain patients, tiotropium-containing triple therapy could be better than two-drug regimens…..Concerns about increased cardiovascular mortality with tiotropium were not borne out in this study.
Corticosteroids: expect 30% to respond to PO steroids. Methylprednisolone IV @60-120mg, PO @40-48 mg/d divided X 10-14d. Prednisone/ Prednisolone PO 40-60mg/d X10-14d. Long-term systemic steroids more harm than good.
• Not all patients with chronic obstructive pulmonary disease exacerbations benefit from oral steroids however, eosinophilia (peripheral eosinophil counts >2%) can be used as a marker for response to steroids (Am J Respir Crit Care Med 2012;186:48)…. This study suggests that eosinophil-directed treatment might allow us to distinguish COPD exacerbations that don’t require steroids and to eliminate unnecessary treatment in as many as 50% of cases. May be particularly helpful in those who are at high risk for steroid side effects (such as diabetics).
Inhaled corticosteroid therapy (ICS): See Inhaled Corticosteroids | See COPD: Combo Tx | ICS’s are of no benefit in slowing the decline in pulmonary function, but airway reactivity is less (less physician visits decr hospitalizations, NEJM 2000;343:1902).
• ICS and long-acting bronchodilators both reduce the frequency of exacerbations in COPD by ~25% (JAMA 2003;290:2301-12). ICS reduce the rate of decline in lung function by 15% (get 50% reduction if stop smoking) (Thorax 2003;58:937-41).
• Recurrent exacerbations in pt’s with moderate-to-severe COPD should be treated with ICS (Eur Respir J 2003;21:1:68-73).
• The recent use of inhaled corticosteroids for asthma or COPD is associated with a dose-related increase in hip fx among older subjects (Am J Respir Crit Care Med 2002;166:1563-1566).
• The use of ICS (triamcinolone 1200 mcg qd) by middle-age or elderly COPD pt’s may lead to increased bruising and impaired skin healing (Chest 2004;126:1123-1133).
• The risk for penumonia was not elevated in COPD patients who received budesonide according to a meta-analysis (Lancet 2009;374:712)…..Budesonide is cleared more rapidly from the airways than fluticasone…the authors also note that no study has shown that fatal pneumonia is more common among patients who receive inhaled steroids, which suggests that pneumonias induced by inhaled steroids are relatively mild…….An editorialist concludes that the benefits of inhaled steroids in COPD patients continue to outweigh the risks substantially.
• ICS does not help reduce all-cause mortality rates in patients with COPD according to a systematic review and meta-analysis (JAMA. 2008;300:2407-2416)…..”ICS therapy is associated with a higher risk of pneumonia.
• Long-term use of inhaled corticosteroids is associated with an increased risk for pneumonia (RR = 1.60) for patients with COPD, according to a meta-analysis (Arch Intern Med. 2009;169:219-229).
• The benefit of adding inhaled corticosteroids to long-acting beta-2 agonists for treating COPD is limited, and furthermore, it’s accompanied by substantial risks of pneumonia (by 63%) and other infections viral respiratory infections by 22% and oropharyngeal candidiasis by 59%) according to a systematic review of 18 RCT’s (Chest 2009;136:1029-1038)……”It is likely that most patients with COPD should be treated only with long-acting beta-2 agonist monotherapy,” at least until future research finds COPD phenotypes most likely to benefit from inhaled corticosteroids.
• Although inhaled corticosteroids (ICS) can help prevent exacerbations of COPD, an analysis of 11 randomized trials with 8164 patients suggests that the benefits have been overstated (Chest 2010;137:318-325)…..On initial analysis, ICS reduced the risk of COPD flare-ups by 18%. Although there was no apparent publication bias, there was significant statistical heterogeneity between the studies……On sensitivity analysis, the benefit of ICS use in preventing exacerbations was confined to patients with a baseline forced expiratory volume in one second (FEV1) of less than 50%. Even in these patients, though, the reduction in risk was only modest (RR, 0.79) and statistical heterogeneity persisted…..Finally, on meta-regression, the benefit of ICS use disappeared completely, regardless of baseline lung function…..”ICS is likely to have only modest benefits in preventing COPD exacerbations, if at all, and should be judiciously used in patients with COPD, keeping in mind the risk-benefit ratio,” the authors conclude……because only 11 studies were included and because the meta-regression considers only mean FEV1 and not the standard deviation, the authors warn that the results should be interpreted with “extreme caution.”
Daliresp (Roflumilast): [500 mcg tab] 1 PO qd without regard to food. Requires several weeks to achieve its effect. Indicated as an add-on therapy to bronchodilators to decrease the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. Clincal trials showed a reduction the rate of moderate or severe exacerbations by 15% and 18%, respectively. The number of exacerbations per patient-year was 1.1 (Daliresp) vs 1.3 (placebo) in one trial and 1.2 (Daliresp) vs 1.5 (placebo) in another trial with the absolute reduction of 0.2 and 0.3 exacerbations per patient-year, respectively. A phosphodiesterase 4 (PDE4, a major cAMP metabolizing enzyme) inhibitor that targets systemic and pulmonary inflammation linked to COPD progression. PDE4 is found in inflammatory cells and is involved in promoting the pathogenesis of COPD. Daliresp suppresses inflammatory mediators, inhibits leukocyte infiltration which attenuated influx of neutrophils and eosinophils into the airways upon endotoxin challenge, potentially slowing the progression of COPD. A selective inhibitor of phosphodiesterase 4 (PDE4). this leads to accumulation of intracellular cyclic AMP in lung cells….reduces neutrophils and eosinophils by 31% and 42%….this give a 15-18% reduction in moderate-severe COPD exacerbations (RR 0.82, ARR 0.2-0.3)
SE: diarrhea (9.5%), weight decrease (7.5%, 20% lost between 5-10% of body weight and 7% lost >10% of body weight), nausea (4.7%), headache (4.4%), back pain (3.2%), influenza (2.8%), insomnia (2.4%), dizziness (2.1%), decreased appetite (2.1%), and abdominal pain (1%-2%). Metabolized by CYP3A4 and CYP1A2, where roflumilast is converted to roflumilast N-oxide via N-oxidation. Co-administration with strong cytochrome P450 inducers (e.g., rifampicin, phenobarbital, carbamazepine, and phenytoin) with Daliresp is not recommended due to potential decreased effectiveness of Daliresp. Forest Pharmaceuticals, Inc. $206 for 30 tablets. Tx with roflumilast (250-500 mcg), a phosphodiesterase-4 inhibitor (anti-inflammatory agent), can improve lung function and reduce flare-ups in pt’s with moderate to severe COPD (Lancet 2005;366:563-571).
Levosimendan: In vitro studies show that treatment with levosimendan can improve the performance of diaphragm muscle fibers from patients with or without COPD (Am J Respir Crit Care Med 2009;179:41-47)…..At sub-maximal activation, this led to an improvement of force generation in both slow and fast COPD and non-COPD diaphragm fibers, by approximately 25%……”levosimendan appears to be the first drug efficiently improving respiratory muscle function.”
Mucolytics: Expectorants and mucolytics may alter the volume of secretions or their composition. Long-term use may help reduce hypersecretions and possibly bacterial counts to help reduce frequency/duration of COPD exacerbations including days of illness & days of Abx use (BMJ 2001;322:1271-4). Usually only helpful for the pt’s with particularly viscous sputum or frequent & severe exacerbations. Consider a home percussion vest to help loosen airway secretions, reduce Abx use and hospitalization (www.theVest.com).
• For patients with COPD, year-long treatment with the mucolytic agent carbocisteine (1,500 mg qd dosed at two 250-mg tablets TID) eased symptoms and was well tolerated according to a randomized study with 354 patients (Lancet 2008;371:2013-2018)……. reduced COPD exacerbations by 25% during 1 year.
• In participants with chronic bronchitis or COPD, treatment with mucolytics was associated with a small reduction in acute exacerbations and a reduction in total number of days of disability (Cochrane Database Syst Rev. 2010;(2):CD001287)……Benefit may be greater in individuals who have frequent or prolonged exacerbations, or those who are repeatedly admitted to hospital with exacerbations with COPD……Mucolytics should be considered for use, through the winter months at least, in patients with moderate or severe COPD in whom inhaled corticosteroids (ICS) are not prescribed.
SSKI (Potassium Iodide): [1g/ml]. Give 50-250mg PO QID as an expectorant. Increases the volume and decr the viscosity of the respiratory tract secretions. If 300g/1000ml give 1-1.5 ml TID-QID. Also used for thyrotoxicosis to inhibit thyroid hormone synthesis.
N-Acetylcysteine (Mucomyst, NAC): Adult: [10ml vials] Mix 2.5-10 mL of 10% NAC with 2.5 mL saline and place mixture (5 mL) in a small-volume nebulizer for aerosol delivery via NEBS q6-12h. For tracheal instillation (intubation) use 20% NAC solution, mix 2-5 mL NAC with 2 mL saline and inject in 2 mL aliquots. Can provoke coughing and bronchospasm. Also used for acetaminophen toxicity. Can also get OTC in 500, 600 and 1,000mg caps.
Peds: 1-10ml of 20% or 1-20ml of 10% solution nebulized q2-6h.
Guaifenesin: take with a full glass of water. Mucinex (600mg BID) is now the only single-ingredient, extended-release guaifenesin on the market in 2003. It’s available OTC. The FDA took the others off the market because the manufacturers failed to submit new drug applications. OTC Mucinex @ 600-1200mg 1-2 BID or Guiatuss, 100mg/5ml elixir @ 600mg PO BID. Decreases the viscosity of secretions. Fenesin @ 600 mg 1-2 BID. Duratuss G 1200mg BID. No benefit of daily 600-mg N-acetylcysteine (x3yrs) added to standard therapy for COPD (Lancet 2005;365:1552-60)….a trend toward a benefit among pt’s who did not use inhaled steroids.
Humibid LA: one tab q12 hours, not to exceed 2 tablets in 24 hours. Contains two expectorants, guaifenesin 600 mg and potassium guaiacolsulfonate 300 mg per tablet.
Magnesium: 1.2g IV over 20min. SE: increased volume of bronchial secretions, bad odor, nausea, bronchospasm. Is a mucolytic to decrease the thickness of pulmonary mucous secretions.
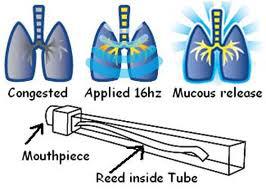 Lung Flute: Uses acoustic wave technology to vibrate sputum loose via a low frequency (16-25Hz) vibration created with a mylar reed inside a clear plastic horn that the patient exhales into. The patient does two sessions 5-10 min per day. FDA approved. Lung Flute for Home Care (includes 14 reeds, approx. 6 month reed supply) @ $45.00. Replacement reed pack (14 reeds) @ $16.50. Via Rx fax at +1 (716) 759-2388 or prescription@medicalacoustics.com or Mail: Medical Acoustics, 9700 Main Street, Clarence, NY, 14031.
Lung Flute: Uses acoustic wave technology to vibrate sputum loose via a low frequency (16-25Hz) vibration created with a mylar reed inside a clear plastic horn that the patient exhales into. The patient does two sessions 5-10 min per day. FDA approved. Lung Flute for Home Care (includes 14 reeds, approx. 6 month reed supply) @ $45.00. Replacement reed pack (14 reeds) @ $16.50. Via Rx fax at +1 (716) 759-2388 or prescription@medicalacoustics.com or Mail: Medical Acoustics, 9700 Main Street, Clarence, NY, 14031.
 A pneumatic vest that provides high-frequency chest-wall oscillation (HFCWO) is a promising therapy for chronic mucus hypersecretion in patients with moderate to severe COPD (European Respiratory Society (ERS) 19th Annual Congress: Abstract P2090. Presented September 14, 2009). The HFCWO vest has several potential advantages. It has been widely used in cystic fibrosis, does not require help from a therapist or caregiver, and the patient can wear it comfortably while seated and simultaneously use respiratory inhalers or watch TV. Subjects were instructed to wear the device twice a day for 15 minutes for a total of 12 weeks…..with a median daily usage of 23.4 minutes for electronic monitoring and of 28.6 minutes for self-report…..Reports of phlegm frequency and severity improved with greater adherence, although there was not a significant effect of adherence on subsequent lung function (predicted FEV1 %) or exercise capacity (6-minute walking test). The data also showed that quality-of-life improvements were mediated by reductions in phlegm rather than improved lung function or exercise tolerance. The mechanism by which mucus hypersecretion contributes to impaired lung function and exacerbation is not known, it might result from plugging in small airways that causes “increased work of breathing and dyspnea,” and/or it might represent a possible V/Q mismatch that causes worsening hypoxemia. Mucus plugging might also enhance bacterial colonization.
A pneumatic vest that provides high-frequency chest-wall oscillation (HFCWO) is a promising therapy for chronic mucus hypersecretion in patients with moderate to severe COPD (European Respiratory Society (ERS) 19th Annual Congress: Abstract P2090. Presented September 14, 2009). The HFCWO vest has several potential advantages. It has been widely used in cystic fibrosis, does not require help from a therapist or caregiver, and the patient can wear it comfortably while seated and simultaneously use respiratory inhalers or watch TV. Subjects were instructed to wear the device twice a day for 15 minutes for a total of 12 weeks…..with a median daily usage of 23.4 minutes for electronic monitoring and of 28.6 minutes for self-report…..Reports of phlegm frequency and severity improved with greater adherence, although there was not a significant effect of adherence on subsequent lung function (predicted FEV1 %) or exercise capacity (6-minute walking test). The data also showed that quality-of-life improvements were mediated by reductions in phlegm rather than improved lung function or exercise tolerance. The mechanism by which mucus hypersecretion contributes to impaired lung function and exacerbation is not known, it might result from plugging in small airways that causes “increased work of breathing and dyspnea,” and/or it might represent a possible V/Q mismatch that causes worsening hypoxemia. Mucus plugging might also enhance bacterial colonization.
The use of available mucolytic agents, like N-acetyl cysteine, is limited by their bad taste and smell. Mechanical approaches like chest physical therapy are time- and labor-intensive and require the services of a trained therapist or caregiver.
The flutter valve, which is a hand-held device with a plastic mouthpiece that the patient exhales into, requires practice, skill, and coordination, and works best with good airflow. Postural drainage, which calls for a head-down position, is difficult for some patients.
Other COPD Meds:
See: Albuterol | Levalbuterol | Anticholinergics | Theophylline |
Anticholinergics –> Ipratropium (Atrovent) 2 puff QID (18ug/puff), 0.02% NEBS @500ug q6-8hr.
Combivent (18 mcg Ipratropium + 90 mcg albuterol MDI with 200 inhalations): 2 puffs QID (max 12 inh/d) Contra: atropine, soybean, lecithin, peanut or related allergy. Atrovent & Combivent (ipratropium) MDI’s contain soy lecithin as a suspending agent (not in the nasal or NEB), thus a contra to anyone with a peanut allergy.
Tiotropium (Spiriva HandiHaler): A bromide powder approved for the long-term, once-daily maintenance tx of bronchospasm associated with COPD. As an anticholinergic drug, it should be used with caution in pt’s with glaucoma and prostatic hyperplasia. Peak effect in 7-8 days. Use albuterol as the rescue inhaler (not ipratropium). 90% with improved FEV 1 changes still present at 24hrs. A long-lasting muscarinic antagonist that has selectivity for M1 and M3 receptors that prevents bronchoconstriction. Costs ~$130 for 30 capsules. Open packet, insert into the HandiHaler, close lid (punctures capsule), then inhale like other dry powder inhalers (repeat inhalation 1x to ensure all of med was inhaled). Qd dosing, superior to Salmeterol and considered first line for COPD (Chest 2002;122:47-55).
DUONEB (Ipratropium 0.5mg = 0.017% + Albuterol 2.5mg = 0.083%): For age >18yo at 1-3ml vial nebulized 4-6X/d. Primarily for COPD.
Beta-2 Agonists:
Albuterol (Proventil, Ventolin) 1-2 puffs q4-6hr (90ug/puff). 0.5% NEB 2.5mg TID-QID.
Metaproterenol (Alupent, Metaprel) PO 10-20mg TID-QID or MDI 2-3 puffs q3-4hr (650 mcg/puff) or 5% NEB 0.2-0.3ml 3-4X/d.
Salmeterol (Serevent) 2 puffs BID (21ug/puff). Adding salmeterol to the existing tx of pt’s with poorly reversible COPD and a history of at least two flare-ups in the last year can improve their sx’s and health status (Thorax 2006;61:122-128).
Terbutaline (Brethaire) MDI 2 puffs q4-6hr (200ug/ puff).
Foradil (formoterol): 1 puff (12 mcg) BID, dry powder, a selective beta2-agonist. “Aerolizer Inhaler”, can be visually inspected so pt’s can confirm they have received the full dose of medicine. In addition, the inhaler makes a whirring noise to indicate that the drug is being dispensed.
Foradil Certihaler (formoterol powder): [60 doses] 1 puff BID. Via the Skyehaler, a novel breath-activated multi-dose dry powder inhaler (MDDPI) device, and Skyeprotect, a powder formulation that protects the drug from atmospheric moisture to ensure product stability and dose-to-dose reproducibility. A long-acting beta-agonist bronchodilator, that combines a rapid onset of action (within 1-3 minutes) with a long-lasting bronchodilation of 12 hours. Caution with mail-order, as heating formoterol capsules to 70°C — the inside temperature of a metal mail box in Phoenix, Arizona — significantly degrades the capsule and results in less formoterol delivered by inhalation (CHEST 2004: Poster 805S. Presented Oct. 27, 2004).
Terbutaline (Brethine, Bricanyl) PO 2.5-5mg q6hr (max, 15mg/d).
Pirbuterol (Maxair) MDI 2 puffs q4-6hr (200ug/ puff).
Methylxanthines:
Theophylline PO 10-12mg/kg/d (200-300mg) QID divided, check level 3-5 days after initiation of therapy. Not beneficial in acute exacerbations.
Aminophylline IV 5.6 mg/kg load, then 0.9 mg/kg/hr per IBW, PO 14mg/kg/d. Check level before additional doses.
Other: Inhalation of furosemide appears to induce bronchodilation and improve exercise-induced dyspnea in COPD pt’s (Am J Respir Crit Care Med 2004;169:1028-1033). The growth-hormone releasing peptide ghrelin (2 mcg/kg IV BID) helps increase food intake and improve body composition in COPD pt’s with cachexia (1kg after 3wks) (Chest 2005;128:1187-1193).
OTC –> Epinephrine (Bronkaid Mist, Primatene Mist) MDI 1-4 puffs q4-6hr. More cardiac and toxic effects than beta-agonists.
Stages, 2007 GOLD Guildelines and Prognosis of COPD:
GOLD = Global Initiative on Obstructive Lung Disease. The spirometric classification of disease severity is based on the post-bronchodilator FEV1 / FVC ratio (<70% establishes the dx). FEV1 determines the degree of severity. Pt’s who started smoking in their 20s and have already sustained appreciable FEV1 loss by their 40s are likely to develop significant COPD if they continue to smoke, but those who have normal FEV1 values, however, probably will not develop symptomatic disease (Am J Respir Crit Care Med 1998;158:49-59).
Stage 1 (Mild): FEV1 >65-80% predicted, FEV1/FVC <70%. Has a minimal impact on quality of life, typically managed by primary care physician, severe hypoxemia not present, no ABG measurement required.
FEV 1 >1L –> good prognosis.
Tx: Ipratropium (Atrovent) 2-6 puffs q6-8hr (max 24puff/24hr) or beta-2 agonist 2 puffs q4-6hr PRN. SE: dry mouth and pharyngeal irritation, Caution if glaucoma, BPH or bladder neck obstruction. Or Salmeterol qHS-BID. Always add PRN short-acting Beta-agonist MDI (max 8-12 puff/24hr) as a “rescue” medication. Patients with GOLD stage 1 COPD may benefit from use of an anticholinergic inhaler (Thorax 2009;64:216-223)…..Compared to use of placebo, ipratropium prompted a 5% increase in FEV1 predicted, a 12% decrease in residual volume predicted and a decrease in specific airway resistance of 81% predicted.
Stage 2 (Mod): FEV1 50-65% predicted. Has a significant impact on health related quality of life, ABG measurements needed periodically.
Tx: May need to add Theophylline, sustained release 200-500 mg BID for nocturnal bronchospasms (aim for level of 8-20mcg/ml or Add Salmeterol (Serevent) 2 puffs BID + mucokinetic. Wheezing and dyspnea on exertion generally occur when the FEV1 is <50% of the predicted value, and significant physical disability usually occurs when the FEV1 is <35-40% of the predicted value.
Stage 3 (Severe): FEV1 <30-50% predicted, profound impact on health-related quality of life. My have h/o respiratory failure or clinical signs of cor pulmonale (get RVH). ABG’s needed, typically managed by a respiratory specialist. Need above tx plus long-term O2 (if low O2 sats).
FEV1 < 0.75 L –> have 30% mortality at 1yr, 95% in 10 yr. Need caution with any surgery. Have easy respiratory compromise, need Abx for URI’s.
Advanced (End-stage, stage 4): O2, bronchodilators (beta + anticholinergic) and pulmonary rehab definitely help. Possible benefit with theophylline, promethazine, Buspirone, inspiratory muscle training and lung-volume reduction surgery. Minimal to no benefit with opioids (oral or inhaled), anxiolytics and antidepressants (West J Med 2001;175:197-201). An inability to “copy drawings with landmarks,” a standard feature of neuropsychological tests, is associated with an increased risk of death in pt’s with severe COPD (Chest 2006;130:1687-1694) (distinctive impairment of executive dysfunction).
Pearls: These somewhat arbitrary cutpoint are “particularly problematic” in elderly pt’s with milder disease because normal aging affects lung volumes. These patients are usually on O2 and have frequent exacerbations. Some specialist have uses Azithromycin 250mg dosing on M-W-Fri to suppress inflammation. Spirometry-based diagnosis and assessment of severity also lumps together emphysema and bronchiolitis along with other different pulmonary phenotypes, and there is evidence that pathological abnormalities of COPD may be present even in the absence of airflow limitation. The clinical diagnosis of COPD is not confirmed in a significant proportion of pt’s at spirometry, suggesting that a more comprehensive approach is required. Stage 0 (“at-risk”) = chronic cough and sputum production, normal Spirometry was eliminated in the 2007 classification of COPD due to incomplete evidence that these individuals necessarily progress on to stage I (Global Strategy for the Diagnosis, Management, and Prevention of COPD: GOLD Executive Summary. Am J Respir Crit Care Med 2007;176:532-555).
Inspiratory-to-total lung capacity (IC/TLC): Reflects static hyperinflation. IC/TLC is an independent risk factor for mortality in pt’s with cCOPD (Am J Respir Crit Care Med 2005;171:591-597) (an IC/TLC <25% doubled the risk of death from any cause, more effectively than dyspnea, six-minute walking distance, arterial oxygen pressure, BMI or level of comorbidity).
The BODE Index: 4-item index. BMI (B), airflow obstruction (O), dyspnea (D) and exercise capacity (E). Get 0-10 point range. A multidimensional grading system that predicts the risk of death from any cause and from respiratory causes in pt’s with COPD more effectively than FEV1 (NEJM 2004;350:1005-12), it includes the body-mass index (0pt if >21, 1pt if <21); FEV1 as a % of the predicted value (0 pt if >65, 1 if 50-64, 2 if 36-49, 3 if <35); the score on the modified Medical Research Council dyspnea scale (0 pt if 0-1, 1 if =2, 2 if =3, 3 pt if =4 = too breathless to leave house or breathless when dressing); and the distance walked in 6 minutes (0 pt if >350meters, 1 if 250-349, 2 if 150-249, 3 if <149). 52-month mortality rates were about 20% in pt’s with scores of 0-2, 30% in p pt’s with scores of 3-4, 40% in pt’s with scores of 5-6, and 80% in those with scores of 7-10.
• Short-term changes in an easily calculated (BODE index) after lung volume reduction surgery (LVRS) for COPD are a powerful predictor of long-term survival (CHEST 2006;129:835,873-878)….a decrease to a lower BODE score class after LVRS correlated with significantly lower mortality (HR = 0.497), compared to pt’s without or with only marginal changes in BODE indexes after surgery.
• The ADO index for predicting a patient’s risk of dying of COPD, appears to perform better than the BODE index according to a study on 232 Swiss and 342 Spanish pt’s (Lancet. 2009;374:667-668, 704-711).
The 2-minute walk test (2MWT): is a reliable and valid test for the assessment of exercise capacity in pt’s with moderate-to-severe COPD (Chest 2006;130:119-125).
Suboptimal control: Prednisone PO 40mg qd for 10-14d, if improve go to qod, then add an inhaled steroid, taper PO steroid slowly.
Ddx: Link: Asthma | Pneumoconiosis |
Obliterative bronchiuolitis: onset in younger age and in nonsmokers. May have a hx of RA or fume exposure. CT on expiration shows hypodense areas.
TB: onset all ages. Lung infiltrations or nodular lesions on CXR. Microbiological confirmation. High local prevalence of TB.
Bronchiectasis: large volume of purulent sputum. Commonly associated with bacterial infections. Course crackles. Clubbing. Bronchial dilation and wall thickening on CXR or CT.
CHF: volume restriction with no airflow limitations of spirometry, basilar crackles, dilated heart. A limited number of items easily available from H&P (h/o CAD, high BMI, laterally displaced apex beat, and raised heart rate), with addition of NT-proBNP and ECG, can help to identify concomitant CHF in individual pt’s with stable COPD (BJU Int 2005;96:1028-1030) (CRP and CXR had limited added value). Pt’s with a dual dx of MI and COPD have twice the risk of mortality, a higher rate of readmission and poorer health status within 1 year after the MI (Am J Cardiol 2007;99:636-641).
LAM: lymphangioleiomyomatosis. A cystic lung dz associated with progressive respiratory failure. Seen in pre-menopausal and those with tuberous sclerosis. Presents with chylothorax/ PTX, see SWISS Cheese on CT. See various section.
Other: CF, Immunoglobins def (IgA, IgG subtypes), Immotile cilia syndrome (Kartagener’s), yellow nail syndrome (lymphedema, pleural effusion, bronchiectasis), sarcoid, HIV.
Ddx of Exacerbations: PE, pneumonia, PTX, pleural effusion, arrhythmia.
Asthma Vs COPD: Despite being a highly treatable disease, asthma is poorly managed in older people because of frequent confusion with COPD (World Allergy Organization (WAO) 2010 International Scientific Conference. Presented December 6, 2010)…..The problem in older people is that there is an overlap of syndromes, both of which give rise to airflow obstruction…..The biology of this hybrid group is poorly understood……”Even high-resolution CT scans don’t necessarily distinguish the difference. We really do need much better diagnostic tests to disaggregate these complex disorders.” …. Using the patient’s history of asthma allows for slightly more precision. “Onset in early life, symptoms that are more variable (although not necessarily), a tendency toward a diurnal pattern for asthma (although not necessarily), and often allergies and comorbidities such as atopic dermatitis are found in asthma but not COPD.” It can still be confusing because patients with asthma can have a family history, but patients with COPD can, too. The onset of symptoms in middle life, a slow progression, a history of smoking, shortness of breath that increases with exercise in a progressive nature, and irreversible airflow obstruction all suggest COPD rather than asthma. A trial treatment with corticosteroids is considered the ultimate test to distinguish asthma from COPD. However, this is not fail-safe……asthmatics who smoke, those with severe asthma, and those with concomitant COPD have symptoms that are all refractory to corticosteroids because of an alteration in the histone deacetylase enzyme, which provides steroid responsiveness…….patients with chronic obstructive or “fixed” asthma show a decline in lung function over time, which is more severe and rapid in patients with nonallergic asthma, often seen later in life. “This type of asthma does not necessarily respond to corticosteroid therapy.” “Functionally, the 2 conditions do become more alike as chronic asthma progresses to fixed airflow obstruction…….The key is to manage patients holistically and assess and treat the individual components of their clinical problems.” “Excessive attachment to inappropriate labeling can lead to some patients being given excessive doses of oral corticosteroids, while others are denied the benefits of anticholinergic drugs or pulmonary rehabilitation because the ‘wrong’ set of guidelines are being followed.”
Surgical: Surgical resection of a large, symptomatic bulla may markedly increase vital capacity and PaO2 by allowing the surrounding normal lung to expand fully (Chest 1996;109:540).
Endobronchial valve: via bronchoscopic approach, on average, four valves per pt were placed. Tx can improve lung function and exercise tolerance in pt’s with end-stage emphysema (CHEST 2006;129:505-506, 518-526). The valve is designed to replicate the effect of surgical long volume reduction by blocking air from entering a portion of the lung. The 90-day mortality rate seen was 1.02% Vs 7.9% for lung volume reduction surgery. An umbrella-style device (IBV Valve) effectively blocks airflow to diseased lung regions in pt’s with emphysema (CHEST 2007;Oct 25)…..Pt’s report an improved quality of life, and the device can be safely removed, if need be.
Lung-Volume-Reduction Surgery (LVRS):
Reduction pneumoplasty. Thought to reduce the hyperinflation and improve the mechanical efficiency of the inspiratory muscles. Clips off areas of hyperinflation to improve diaphragm function to increase elastic recoil and decompress the residual lung. Surgery is still undergoing tx trials. High operative mortality, no benefit in high risk group. 1/3 are long-term responders, ½ die within 4yrs. Candidates include postbronchodilator@ FEV1 <40%, DLCO <50%, TLC >100%, PaCO2 <50mmHg, age <70yo (NEJM 2001;345:1075).
• LVRS leads to significant improvement in dyspnea, lung function, and quality of life that persists for at least 5 years (Chest 2003; 975-976, 1026-1037). LVRS does not confer a survival advantage over and is costly compared to medical therapy (cost-effectiveness of this procedure at 3 years averaged US$190,000 per quality-adjusted life-year gained) (NEJM 2003;348:2059-2102).
• LVRS is more effective in improving quality of life, dyspnea, pulmonary function and exercise tolerance in pt’s with emphysema than medical therapy (Chest 2005;127:1166-1177) (the procedure does not extend life) (FEV1 & walking distance). Although it is associated with a significant mortality risk, LVRS can improve the health status in some pt’s with severe emphysema (Chest 2005:128:3489-3499)…..only a fairly small percentage of all emphysema pt’s who are suitable for LVRS. Up to 20% of emphysema pt’s may be candidates for lung volume reduction surgery…a low exercise capacity at baseline was predictive of the greatest improvements.
• Conversely, pt’s with widespread or lower lobe disease and a higher exercise capacity did the worst and probably should not undergo the operation (Feb 03, 2006 42nd annual meeting of the Society of Thoracic Surgeons). Lung volume reduction surgery (LVRS) reduces the frequency of COPD exacerbations by 30% (Am J Respir Crit Care Med 2008;177:130-131,164-169).
Lung Transplant: A survival advantage has been reported for patients with cystic fibrosis and pulmonary fibrosis. This advantage has not been demonstrated for patients with emphysema.









