11.2 Connective tissue and the pudendal nerve in chronic pelvic pain
Connective tissue dysfunction in chronic pelvic pain
Mechanisms of development of subcutaneous panniculosis
Superficial to muscles with myofascial trigger points
Dermatomes of inflamed neural structures
Superficial to areas of joint dysfunction
Connective tissue restrictions and altered neural dynamics
Efficacy of connective tissue mobilization
Connective tissue manipulation
The pudendal nerve in chronic pelvic pain
Possible consequences of pudendal neuralgia
Connective tissue dysfunction in chronic pelvic pain
Several different terms have been used to describe connective tissue restrictions and/or dysfunction. According to orthopaedic physician Robert Maigne, cellulagia is ‘defined as neurotrophic manifestations that include subcutaneous tenderness and thickening’. It can be detected by using the ‘pinch-roll’ test, in which a fold of skin is rolled between the fingers causing pain, with the clinician noting thickening (Maigne 1995) (Figure 11.2.1).
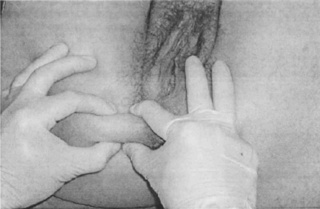
Figure 11.2.1 • Skin rolling test: the skin of the perineum is pinched just below the level of the anus and rolled to the front searching for a sharp pain at one level (Beco 2004)
Physical therapist Maria Ebner (1985) used the term trophic oedema to describe thickened hypersensitive loose connective tissue. Other literature sources have referred to dermographia, which is defined as a condition in which pressure or friction of the skin gives rise to a transient reddish mark so that a line on the skin becomes visible (Merck Manual 2008). Finally, the terms panniculosis and fibrositis have also been used to describe dysfunctional connective tissue (Travell & Simons 1993), defined as inflammatory hyperplasia of the white fibrous tissue. This text will use the term subcutaneous panniculosis to describe thickened connective tissue that is tender upon pinch rolling (i.e. dysfunctional connective tissue).
In addition to presenting as thickened or dense upon skin rolling, areas of subcutaneous panniculosis may show vasomotor, pilomotor and sudomotor reactions, increased subcutaneous fluid and atrophy or hypertrophy of the underlying muscles (Chaitow 2010). Underlying muscle atrophy is the resultant effect of the thickened tissue interfering with proper functioning of sodium–potassium pumping mechanisms in muscles (Ebner 1975). Panniculosis can cause local nociceptive pain via the peripheral nervous system, and it is hypothesized to cause referred pain in distant locations including the viscera through the central nervous system (Bischof & Elmiger 1963).
Mechanisms of development of subcutaneous panniculosis
When connective tissue becomes dysfunctional the problems that arise are in proportion to the support the tissues provide when they are healthy. Several mechanisms have been identified to explain how dysfunction develops in the subcutaneous tissue. These are as the result of visceral referred pain, in tissue superficial to myofascial trigger points, in the cutaneous distribution of inflamed peripheral nerves, and superficial to or referred from areas of joint dysfunction (Ebner 1975, Travell & Simons 1993, Beco 2004, Maigne 1996).
Viscerosomatic reflex
The viscerosomatic reflex is a reflex in which somatic manifestations occur in response to visceral disturbances. More specifically, the visceral-cutaneous reflex is a phenomenon where disturbances or disease in visceral organs refer pain along the distribution of somatic nerves which share the same spinal segment as the sensory sympathetic fibres to the organ affected (Head 1893). The visceral-cutaneous reflexes have been studied by many and are commonly referred to as Head’s zones, Chapman’s reflexes, or Mackenzie’s zones (Beal 1985) (see Figures 11.2.2, 11.2.3). The reflex is initiated by afferent impulses from visceral receptors, impulses travel to the dorsal horn of the spinal cord, synapse with interconnecting neurons, connect with sympathetic and peripheral motor efferents resulting in sensory changes in the blood vessels and skin (and also muscle and viscera) (Bischof & Elminger 1963). If the pathological visceral afferent stimulation becomes chronic, neurogenic plasma extravasation will occur in the skin, thereby causing vasoconstriction in the periphery, hyperaesthesia and thixotropic changes. In more recent literature, three plausible neural mechanisms have been identified in animal models to explain visceral-cutaneous reflexes.
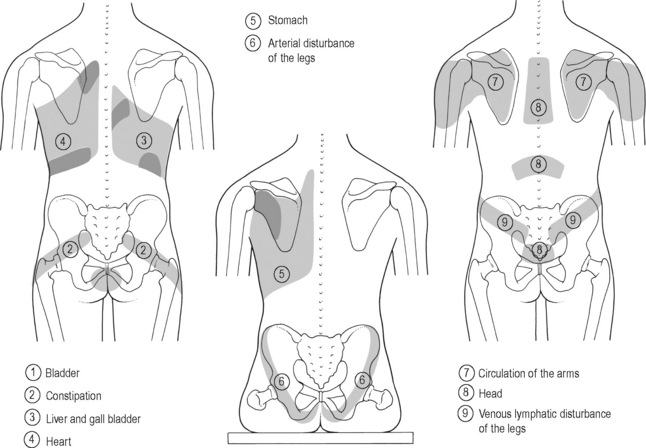
Figure 11.2.2 • Head’s zones.
Adapted from Chaitow (2003) Modern Neuromuscular Techniques, second ed, Elsevier.
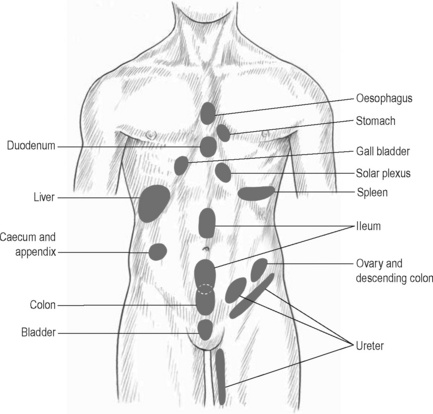
Figure 11.2.3 • McKenzie’s zones.
Adapted from Chaitow (2003) Modern Neuromuscular Techniques, second ed, Elsevier.
In 1996 Takahashi & Nakajima published that plasma extravasation occurred as a result of antidromic stimulation of C-fibres in spinal nerves. It is noted that with unilateral stimulation changes in the skin can occur unilaterally or bilaterally (Takahashi & Nakajima 1996).
Studies suggest that the actual mechanism may be a combination of the three described processes, as noted by Ursula Wesslemann et al. in 1997. One potential mechanism is described because dichotomizing sensory neurons have a branch to both the uterus and to the skin. Uterine inflammation could cause excitation of the visceral branch of the afferent neuron, leading to antidromic activation of the somatic branch causing neurogenic plasma extravasation. Wesselmann also hypothesized that visceral afferent neurons may excite cutaneous afferent neurons. The result of this spinal mechanism is antidromic activation of cutaneous afferent fibres, again resulting in plasma extravasation. Finally, the paper discussed the possibility that sympathetic post-ganglionic nerve terminals must be intact. This was demonstrated by a decrease in plasma extravasation when the anterior spinal root was not intact (Wesslemann & Lai 1993).
Similarly, basic science studies (Beal 1985, Craggs 2005) have shown that somatic disturbances cause visceral changes, otherwise known as the somatovisceral reflex.
Superficial to muscles with myofascial trigger points
Travell & Simons (1993) have reported a strong association between active myofascial trigger points and subcutaneous connective tissue restrictions. Dermographia/fibrositis commonly occurs most often over muscles of the back of the neck, shoulders and torso, and less frequently over limb muscles. In panniculosis, the subcutaneous tissue exhibits increased viscosity suggestive of thixotropy. It is proposed by Travell and Simons that the connective tissue restrictions may be related to sympathetic nervous system activity involving mechanisms operating in the underlying myofascial trigger points. Treating the panniculosis can relieve myofascial trigger point activity and/or make the underlying myofascial trigger point more responsive to treatment. Travell and Simons identify the need for a well-designed study to critically evaluate the relationship between myofascial trigger point activity and the presence of overlying panniculosis.
Dermatomes of inflamed neural structures
The ‘pinch-roll’ test (of the subcutaneous tissue in the territory of a peripheral nerve) is commonly accepted as a clinical indicator of inflamed neural tissue. Referred pain is accompanied by hyperalgesia of the skin and subcutaneous tissues in the involved dermatomes. This hyperalgesia or hypersensitivity can be revealed by gently grasping a fold of skin between the thumbs and forefingers, lifting it away from the trunk and rolling the subcutaneous surfaces against one another in a pinch and roll fashion. The entire dermatome may be affected or only partial tissue changes may be seen (Maigne 1996, Beco 2004).
Superficial to areas of joint dysfunction
Robert Maigne (1995, 1996) coined the term ‘cellulagia’ when reporting that intervertebral joint dysfunction causes neurotrophic reflexes. This cellulagia can occur in the skin innervated by the corresponding nerve roots and in tissue superficial to areas of vertebral dysfunction.
The dysfunctional tissue itself and the sequellae associated with subcutaneous panniculosis can perpetuate chronic pelvic pain (CPP) and dysfunction. Subcutaneous panniculosis can cause local nociceptive pain, hypothesized visceral referred pain through the central nervous system, underlying muscle dysfunction and altered neurodynamics. The thickening of the skin causes ischaemia and therefore nociceptive pain via the peripheral nervous system (Holey 1995). In addition to local pain, the presence of increased subcutaneous fluid will cause an alteration in the osmotic pressure in cells. There is retention of sodium and associated excretion of potassium, resulting in water retention that interferes with the neuromuscular conducting mechanism (Ebner 1975). The physiological consequence of this is underlying muscle atrophy, which may then lead to the development of myofascial trigger points, a further source of pain and dysfunction. Multiple literature sources describe the mechanisms of which subcutaneous panniculosis can perpetuate visceral disturbance and that the association between cutaneous dysfunction and visceral disturbance is so high that examination of the skin can be used as a predictor of potentially undiagnosed visceral disease (Korr 1949, Wilson 1956, Grainger 1958, Beal 1985, Tillman & Cummings 1992).
Connective tissue restrictions and altered neural dynamics
As discussed below, a peripheral nerve is vulnerable to neural dynamics if its blood supply and/or normal neurobiomechanics are compromised along its path. Ischaemia or thickness associated with subcutaneous panniculosis can compromise neural gliding mechanisms, particularly when the peripheral nerves innervate or transect the dysfunctional tissue region (Butler 2004).
Any muscle and/or tissue and/or structure innervated by an affected nerve may begin to generate pain (Butler 2004). For example, a patient with connective tissue restrictions in the territory of the pudendal nerve may experience sharp, stabbing vaginal or urethral pain with increasing degrees of hip flexion. This is because the nerve must lengthen when the hip flexes; if the tissue is restricted the nerve will not be able to lengthen and the adverse tension results in neuralgic pain in the territory of the nerve. Consequently, because the and/or perineal portions of the pudendal nerve innervate the distal third of the urethra a patient may subsequently feel urethral burning. In terms of bowel function, it is not uncommon for patients with CPP to experience constipation. Connective tissue restrictions affecting the pudendal nerve can also cause neuralgia symptoms, as the restrictions will restrict the neural mobility required for lengthening when a person strains. As a result, a patient may feel neuralgic symptoms in the territory of the nerve, either immediately or with a delayed onset (Holey 1995).
The matrix of areolar connective tissue also serves to deposit collagen for the formation of scar tissue. Commonly, women with CPP have undergone laparoscopic investigation as an attempt to identify pain generators. The trochar (a surgical instrument) may have been used through the umbilicus, in the suprapubic region, or other lower abdominal sites. Formation of scar tissue here can directly create restriction of the ilioinguinal, iliohypogastric and genitofemoral nerves (Howard 2000). Peri-umbilical and suprapubic subcutaneous panniculosis secondary to incisions have been associated with urinary urgency, frequency and dysuria (Fitzgerald & Kotarinos 2003).
Efficacy of connective tissue mobilization
Over the last 20 years basic science research has confirmed the interaction between muscle, skin, viscera and central and peripheral nervous systems supporting the importance of addressing connective tissue as part of any pain-related treatment programme. Recent clinical research shows the physiological and clinical benefits of CTM (Kaada & Torsteinbo 1989, Brattbert 1999, Maddali-Bongi et al. 2009, Fitzgerald et al. 2009).
The Urological Pelvic Pain Collaborative Research Network and the National Institutes of Health examined the feasibility of conducting a randomized clinical trial to compare two methods of manual therapy, external and internal myofascial physical therapy (MPT), compared to traditional external global therapeutic massage (GTM) among patients with urologic CPP syndromes (Fitzgerald et al. 2009). Connective tissue manipulation was the primary external myofascial technique in the MPT group. They were able to standardize both treatment approaches. They found the MPT group had a response rate of 57% which was significantly higher than the rate of 21% in the GTM treatment group (P = 0.03). The overall response rate of 57% in the MPT group suggests that MPT represents a clinically meaningful treatment option. We can infer from these results that there is clear evidence of benefit of connective tissue manipulation in patients with myofascial pelvic pain and dysfunction.
One recent study evaluated the efficacy of a rehabilitation programme based on the combination of connective tissue massage and McMennell joint manipulation specifically for the hands of patients suffering from systemic sclerosis (Maddali-Bongi et al. 2009). In the 40 patients enrolled, 20 (interventional group) were treated for a 9-week period with a combination of connective tissue massage, McMennell joint manipulation and a home exercise programme, and 20 (controlled) were assigned only to a home exercise programme. The interventional group improved in multiple functional and quality of life tests at the end of the treatment (P < 0.0001) versus the control group. Therefore, they concluded that the combined treatment may lead to an improvement in hand function and quality of life.
In 1999 Brattberg investigated the effect of connective tissue massage in the treatment of patients with fibromyalgia. He randomized 48 individuals diagnosed with fibromyalgia, 23 in the treatment group and 25 in the reference group. After a series of 15 treatments of CTM he found that the treatment group reported a pain-relieving effect of 37%, reduced depression and the use of analgesics, and positive effects in their quality of life (Brattbert 1999).
In 1989, another study examined the concentration of plasma beta-endorphins in 12 volunteers before and 5, 30 and 90 minutes after a 30-minute session of CTM. They found a moderate mean increase of 16% in beta-endorphin levels from 20.0 to 23.2 pg/0.1 ml (P = 0.025) lasting for approximately 1 hour with a maximum in the test 5 minutes after termination of the massage. They concluded that the release of beta-endorphins is linked with the pain relief and feeling of warmth and well-being associated with the treatment (Kaada & Torsteinbo 1989).
Connective tissue manipulation
The original technique described by Dicke (1953) and more recently by Ebner (1975) and Holey (1995) involves particular strokes in very specific directions and patterns throughout the entire body depending on the pathology. The authors’ use of CTM is based upon Dicke’s technique in theory and practice, but the technique has been modified for the CPP population specifically. In this text, CTM will be described as the authors utilize it in practice, which has not been described previously.
Evaluation
• Severity of connective tissue (CT) restrictions correlate to severity of symptoms;
• Mild tissue restrictions will cause slight tissue irritation when compressed for long periods;
• Moderate restrictions may cause a hypersensitivity to touch;
• Severely restricted tissue can cause pain without touch, stretch or compression and/or skin fissures.
• Small amount of massage cream;
• Tissue assessed by rolling tissue between tips of thumbs and fingers;
• Thumbs slide underneath CT while fingers grasp tissue and pull towards thumb;
• Tips of fingers used, not pads, therefore short fingernails are required;
• Grasp is firm and fairly superficial;
• Pressure to skin is minimal;
• Direction of force is parallel to tissue, not perpendicular;
Less restricted tissue is easier to mobilize, more restricted tissue is more difficult:









