Chapter 7 Complications Related to Radiofrequency Procedures for the Treatment of Chronic Pain
 RFA can allow for reliable and discrete thermal lesioning in a desired specific neurologic location with prolonged pain relief.
RFA can allow for reliable and discrete thermal lesioning in a desired specific neurologic location with prolonged pain relief. Severe complications are rare but can be as catastrophic as heart block and permanent spinal cord or brain injury.
Severe complications are rare but can be as catastrophic as heart block and permanent spinal cord or brain injury. Adequate training and board certification of the interventional physician are ideal and could theoretically lead to greater safety measures and less chance of complications when RFA is used.
Adequate training and board certification of the interventional physician are ideal and could theoretically lead to greater safety measures and less chance of complications when RFA is used. The ability to implement lifesaving measures such as intubation, advanced cardiac life support, and management of local anesthetic toxicity as well as an extensive knowledge of the anatomy and proficiency at reading fluoroscopic images are mandatory.
The ability to implement lifesaving measures such as intubation, advanced cardiac life support, and management of local anesthetic toxicity as well as an extensive knowledge of the anatomy and proficiency at reading fluoroscopic images are mandatory. Consent; sterile technique; and a review of the patient’s allergies, use of anticoagulants, and medical history (especially infection risks and indwelling pacemakers, defibrillators, or spinal cord or peripheral nerve stimulators) are essential.
Consent; sterile technique; and a review of the patient’s allergies, use of anticoagulants, and medical history (especially infection risks and indwelling pacemakers, defibrillators, or spinal cord or peripheral nerve stimulators) are essential. General anesthesia should be avoided; light sedation via the guidelines set forth by the American Society of Anesthesiologists for conscious sedation and monitoring can be used by properly trained personnel, but it is recommended that the patient be able to maintain meaningful communication throughout the entire procedure.
General anesthesia should be avoided; light sedation via the guidelines set forth by the American Society of Anesthesiologists for conscious sedation and monitoring can be used by properly trained personnel, but it is recommended that the patient be able to maintain meaningful communication throughout the entire procedure. Use of negative aspiration; myelogram-compatible contrast under live fluoroscopy; and when indicated, a test dose with local anesthetic and dilute epinephrine can mitigate risks.
Use of negative aspiration; myelogram-compatible contrast under live fluoroscopy; and when indicated, a test dose with local anesthetic and dilute epinephrine can mitigate risks. Evaluation of specific motor stimulation and, when applicable, sensory stimulation with their respective relevant responses should always be used.
Evaluation of specific motor stimulation and, when applicable, sensory stimulation with their respective relevant responses should always be used. Severe complications associated with RFA of the cervical, thoracic, and lumbar medical branches include but are not limited to pneumothorax and permanent nerve or spinal cord injury.
Severe complications associated with RFA of the cervical, thoracic, and lumbar medical branches include but are not limited to pneumothorax and permanent nerve or spinal cord injury. Severe complications associated with RFA of the sympathetic ganglion and nerves include but are not limited to heart block, total spinal blockade, pneumothorax, meningitis, major vessel injury (vertebral artery, subclavian vessels, vena cava, or aorta), blindness, spinal cord injury, and brain injury.
Severe complications associated with RFA of the sympathetic ganglion and nerves include but are not limited to heart block, total spinal blockade, pneumothorax, meningitis, major vessel injury (vertebral artery, subclavian vessels, vena cava, or aorta), blindness, spinal cord injury, and brain injury.Introduction
Radiofrequency (RF) has been used clinically as a modality to treat chronic pain as early as the 1950s.1,2 It is unique in that it can allow for reliable and discrete thermal lesioning in a desired specific neurologic location. The end result can produce pain relief from a prolonged duration up to 6 months or longer. RF is a low-energy, high-frequency, alternating current of which an electrical field is created between the active electrode and the grounding pad. The term radio was implemented because the frequency field that is used for treatment is often within the same frequency range of AM radio (300 to 500 kHz).3 When the RF probe and dispersive grounding pad are properly placed, the body tissue completes the circuit. The uninsulated needle tip is the active portion of the electrode. The probe itself does not generate heat; rather, the frictional dissipation of current within the surrounding tissue heats the electrode.4
Radiofrequency can either be continuous at a temperature usually greater than 45°C or intermittently dispersed at 42°C; the latter is referred to as pulsed RF. Continuous RF ablation (RFA) is considered to be neurodestructive. Pulsed RF therapy is not fully understood but is thought to be a type of neuromodulation.5 The active tip of the RF probe is beneficial as it also can be used for monitoring electrical impedance, sensory and motor stimulation, and real-time temperature measurement and control. The RF technique virtually eliminates undesired results such as combustion, sticking and breaking of the probe, and charring of the adjacent tissue. Other neuroablative techniques such as chemical destruction and cryotherapy do not have this luxury and are less predictable with the lesioning size. Even the use of a laser for neurodestruction has apparent limitations in creating an effective lesion compared with RF therapy.6 Although conventional RFA for the treatment of chronic pain has proven to be an effective minimally invasive treatment option, it is not without its inherent risks and complications.
Closed Claims Cases in Pain Management and Ablative Procedures
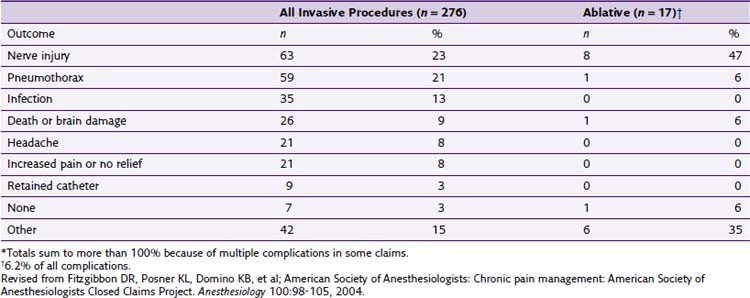
The American Society of Anesthesiologists (ASA) Closed Claims Project in 2004 by Fitzgibbon et al7 reported an increase in adverse chronic pain outcomes from 1979 to 1999. During the 1980s, there was a 3% increase in chronic pain claims. It increased to 10% in the 1990s. Fortunately, most of the chronic pain management claims were temporary or nondisabling injuries. Of the 276 claims related to chronic pain invasive procedures, 17 were associated with ablative procedures (chemical or thermal), making up 6.2% of the overall claims. Interestingly, only four cases were related to temperature (thermal RF or cryoablation) complications (Table 7-1).
Table 7-1 Procedures in Chronic Pain Management Claims, 1970-1999 (n = 276)
| Claims | Number | Percentage |
|---|---|---|
| Invasive procedures | 276 | 100 |
| Injections | 138 | 50 |
| Epidural steroids ± local anesthetic agents | 114 | |
| Trigger point | 17 | |
| Facet | 4 | |
| Other | 3 | |
| Blocks | 78 | 28.3 |
| Peripheral | 28 | |
| Stellate ganglion | 19 | |
| Other autonomic | 9 | |
| Neuraxial | 9 | |
| Upper or lower extremity | 7 | |
| Axial | 4 | |
| Head and neck | 2 | |
| Ablative Procedures | 17* | |
| Chemical (phenol, alcohol) | 13 | |
| Temperature (RF, cryoablation) | 4 | |
| Implantation or Removal of Devices | 12 | 4.3 |
| Implantable pump | 5 | |
| Nerve stimulator | 4 | |
| Catheter | 3 | |
| Device Maintenance | 20 | 7.2 |
| Other Interventions | 11 | 4 |
RF, radiofrequency.
Revised from Fitzgibbon DR, Posner KL, Domino KB, et al; American Society of Anesthesiologists: Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology 100:98-105, 2004.
One of the largest confounding variables of the Closed Claims Project is that it primarily selects for cases that are of a greater severity and injury. Pursuit of cases that will likely yield less than $50,000 is not typically characteristic of plaintiff attorneys.8 Therefore the amount of overall untoward events and complications are more than likely greater because they are not recorded from a legal standpoint or simply not reported by the pain practitioner. Thirteen of the 17 closed claims were related to chemical ablation. It is clear that chemical ablation seems to carry a higher risk of severe complications. Unfortunately, the ASA project did not further delineate between which specific complications were related to chemical versus temperature-related neurolysis (Table 7-2).
Avoidance of Radiofrequency Complications
Patient Selection Factors
Absolute contraindications include a lack of patient consent, infection at the site of RF probe placement, systemic infection, anticoagulants,* pacemaker or implantable cardioverter defibrillator (ICD),* and nonsystemic infection distant from the RF site.*
Relative contraindications include active comorbid disease, pregnancy,* poor hygiene, allergy to pertinent RF-related medication (local anesthetic, iodine, latex), anticoagulants,* pacemaker or ICD,* preexisting dorsal column or peripheral nerve stimulator,* and nonsystemic infection distant from the RF site.*
Sedation
Conscience sedation or monitored anesthetic care during chronic pain procedures is a topic of debate among experts in the field of pain medicine. Some believe that sedation should be avoided in procedures that have an inherently higher risk of catastrophic outcome if injury to vascular or neurologic structures were to occur (e.g., cervical epidural steroid injection, stellate ganglion [SG] block). It is thought that local anesthetic only would allow for the patient to communicate marked pain related to ensuing injury to vital structures.9 The opposing view is that if a patient has light sedation via the guidelines set forth by the ASA for conscious sedation and monitoring as well as implemented by the hands of an experienced anesthesia provider, he or she may have a more comfortable experience and is thus less likely to make sudden movements. Sudden patient movement during the procedure could significantly affect the outcome of the procedure and potentially increase the chance of an untoward event. The anesthetic goal for advocates of light sedation is to have a minimally anxious patient who is able to maintain meaningful communication throughout the entire procedure and stable vital signs. In either case, it is at the discretion of the pain and anesthesia providers to decide which patients are good candidates. Light sedation with agents that can be reversed are recommended (benzodiazepines: midazolam; short-acting opioids: fentanyl). A propofol anesthetic would be less than ideal because of the chance of (1) the patient becoming disinhibited during the procedure10 and (2) unintentional general anesthesia could prevent the patient from alerting the physician to complications. In the United States, general anesthesia for common percutaneous chronic pain procedures is thought to be excessive and risky by most pain specialists.
Infection
Although there have been no literature reports of infection complications with RF procedures, pain practitioners should minimize this risk by postponing RF treatment with the presence of active infection or concurrent antibiotic or antimicrobial use. Consultation with an infectious disease specialist should be considered if the patient has a history of osteomyelitis or methicillin-resistant Staphylococcus aureus. Studies have shown that smoking cessation promotes better healing in surgical patients.11 Although no studies have linked the abatement of smoking with patients undergoing percutaneous RFA, the practitioner may want to take this into consideration if the patient is at high risk for infection (e.g., history of infection, abscess). Providers may also want to instruct patients with poor hygiene to bathe with over-the-counter Hibiclens (4% chlorhexidine) the night before the procedure. This may decrease the chance of infection by the seeding of human skin flora via percutaneous technique.12
Cheng and Abdi13 describe infection complications from several different case reports with patients undergoing intraarticular zygapophyseal (facet) joint injections. These complications include epidural abscess, septic arthritis, spondylodiscitis, and chemical meningism (thought to be related to inadvertent dural puncture with a high-dose steroid injection). These authors also report no known case reports of infections related directly to RF neurolysis; however, one could reasonably conclude that the above complications could occur with RF if confirmation of proper needle placement and sterile precautions are not used.13
Presence of a Pacemaker or an Implantable Cardioverter-Defibrillator Device
Radiofrequency in patients with a cardiac pacemaker or ICD is a controversial topic. Some authors believe that it is adequate to turn off the pacemaker before proceeding with RF lesioning.14 However, the routine practice of placing of a magnet to set the pacer to an asynchronous mode should be reconsidered because of the ever-changing complexity of cardiac pacemakers. A comprehensive pre- and postprocedure evaluation should be performed by the device representative as well as intraoperative monitoring during the RF procedure itself.15 This practice is supported by the 2002 American College of Cardiology and American Heart Association (ACC/AHA) guideline update for perioperative cardiovascular evaluation for noncardiac surgery.16 Consulting the patient’s cardiologist is advised, but one should be aware that there has been inaccurate information about pacer function given by cardiologists;17 therefore the presence of the cardiac device representative is still advised. RFA procedures should never be done while the defibrillator function is still active, regardless of the location of the RF lesioning in the body. There are known medicolegal cases involving patients being defibrillated after RF treatment was started. There have been anecdotal reports of RF lesioning being done safely for pain patients with indwelling defibrillators, but this was done after the defibrillator function was deactivated and the ACC/AHA guidelines used; the formal literature is lacking. In the rare case that the pain practitioner decides to move forward with an RF procedure, it is advised to use extreme caution with meticulous attention to the cardiologist’s direction and complete compliance with the ACC/AHA guidelines.
Anticoagulation
The American Society of Regional Anesthesia Consensus Guidelines for the anticoagulated patient by Horlocker et al18 have become the gold standard when performing neuraxial procedures in anticoagulated patients. Interventionalists should have a thorough understanding of these guidelines, anticoagulant medications, and the nuances of pathology associated with a deviation from the normal clotting cascade. Adherence to these guidelines is not a fail-safe measure, and the pain specialist should always exercise vigilance when there is the possibility of an epidural hematoma.
A traumatic procedure seems to be the biggest risk factor in epidural hematoma formation.19 The incidence of a hematoma when entering the epidural space is estimated to be one in 20,000 (traumatic) to one in 220,000 (nontraumatic).18,20 Because of the nature of the common RF spine procedures, inadvertent needle placement in the epidural space is certainly possible. Because the epidural space is not accessed during most RF procedures, it is thought that the incidence of epidural hematoma would be much lower than with epidural steroid injections. No case reports in the literature have been associated with RFA and epidural hematoma formation. However, there have been epidural hematomas with surgical evacuation after intraarticular facet joint injections for pain.21 The larger gauge needles used in RF procedures could have a higher propensity to cause severe bleeding if cessation of anticoagulation medications is not done. As a general rule for outpatient elective chronic pain procedures, an international normalized ratio (INR) of less than 1.3 and a platelet count greater than 100,000 (assuming no qualitative platelet dysfunction such as in end-stage renal disease) is desired, although some practitioners use an INR of less than 1.5 and platelets greater than 80,000 if the patient is asymptomatic.
Physician-Dependent Factors
Infection or Abscess
Poor sterile technique by the pain physician or the procedural team can certainly increase the risk of infection or abscess formation. Surgical masks can contribute to a decrease in morbidity related to infection.12 A surgical scrub and prep with chlorhexidine with ethanol (CHE) has been shown in studies to be superior to iodine-based solutions.22 Viable microorganisms of the human skin flora were still present on the skin of patients after preparation with iodine-based products to a level of significance (P <.01) compared with CHE.23 Proper precautions by the procedural team as well as the above suggestions will decrease the likelihood of complications related to infection.
Faulty Equipment
A careful examination of the insulation of the RF needles should always be done. To date, there have been four reported cases of superficial burns secondary to disrupted insulation of the probe.24 Kornick and associates25 described two case reports of burns related to a malfunction of the adhesion site of the grounding pad.
Radiofrequency Probe Placement and Neurolysis
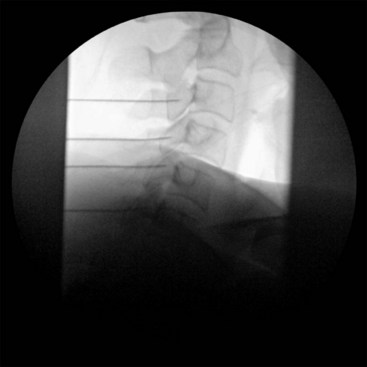
Fluoroscopic guidance when placing the RF needle is essential to ensure patient safety and mitigate complication risks. As mentioned above, neurologic injury was the most common severe complication related to neuroablative procedures in the ASA Closed Claims Project cases. Pneumothorax was also an issue. The application of an extensive knowledge of the anatomy along the trajectory of the needle will minimize risk to the nerves, viscera, and vascular structures. It is imperative that the fluoroscopic images have no parallax. Sharp alignment of the margins of the bilateral osseous target structures minimizes confusion of where exactly the needle is being placed. Fig. 7-1 is a lateral fluoroscopic image that demonstrates a malalignment or parallax of bilateral osseus structures (C5) and an optimized or crisp alignment of structures (C3) in the same image; parallax of the target area increases the risk of incorrect needle placement and possible neurologic injury.
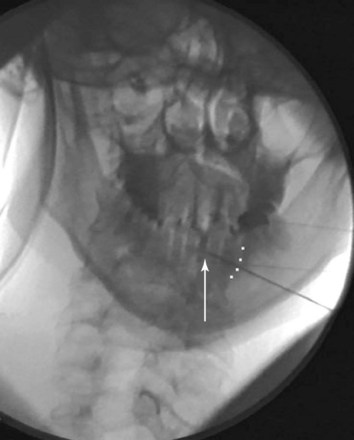
Contrast dye injected under live fluoroscopy should always be used if the needle tip is to be placed in the vicinity of a vascular structure or certain vital organs (e.g., lung, bowel). There have been no formal case reports related to RFA and nerve or viscera injury; however, Bogduk and associates26 report two medicolegal cases involving direct spinal cord trauma. The first case of spinal cord injury involves a patient undergoing a C3–C4 RFA. The case was done under general anesthesia. The proceduralist placed the active tip of the RF probe medial to the facet joint, traversed the lamina, and into the spinal cord. This was confirmed via the intraoperative images. The patient developed Brown-Séquard syndrome subsequent to the procedure. The two key points are that (1) general anesthesia rendered the patient helpless in being able to report symptomatology related to impending cord injury and (2) the radiographic images were not properly used to ensure correct needle placement. The second case involved a patient who underwent an attempted C3 (third occipital) RFA. The anteroposterior (AP) images showed the RF probe was placed at the wrong level (C3–C4). The tip was also too medial and anterior and thus inside the foramen (Fig. 7-2); the result was severe neurologic injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree