Complication
Diagnosis of problem
Treatment of problem
Neuraxis complications
Nerve injury
CT or MRI, EMG/NCS/physical exam
Steroid protocol, anticonvulsants, neurosurgery consult
Epidural fibrosis
Increased stimulation amplitude
Lead reprogramming, lead revision
Epidural hematoma
Physical exam, CT or MRI
Surgical evacuation, steroid protocol
Epidural abscess
Physical exam, CT or MRI, CBC, blood work
Surgical evacuation, IV antibiotics, Infectious Disease consult
Post–dural puncture headache
Positional headache, blurred vision, nausea
IV fluids, rest, blood patch if required
Device complications
Unacceptable programming
Lack of stimulation in area of pain
Reprogramming of device, revision of leads
Lead migration
Inability to program, x-rays
Reprogramming, surgical revision
Current leak
High impedance, pain at leak site
Revision of connectors, generator, or leads
Generator failure
Inability to read device
Replacement of generator
Nonneurological tissue complications
Seroma
Serosanguinous fluid in pocket
Aspiration; if no response, surgical drainage
Hematoma
Blood in pocket
Pressure and aspiration; surgical revision
Pain at generator
Pain on palpation
Lidoderm patches, injection, revision
Wound infection
Fever, rubor, drainage
Antibiotics, incision and drainage, removal
15.1 Complications of the Neuraxis
15.1.1 Epidural Hematoma
Bleeding in the epidural space is common when needles and leads are introduced. In most patients, this bleeding is unnoticed and causes no sequelae. In rare patients, the bleeding progresses to the development of an epidural hematoma. If a developing epidural hematoma progresses, it can lead to numbness, back and leg pain, weakness, and eventual paraplegia. The treatment for clinically significant epidural hematoma is surgical evacuation. It is critical that this problem be identified early and treated within 24 h of the development of symptoms. Weakness in the postoperative period after device implantation is a red flag warning that should raise the suspicion of this tragic complication.
Risk factors for developing an epidural hematoma include the use of anticoagulants, platelet-acting drugs, aspirin, or NSAIDs. Independent risk factors for epidural hematoma following spinal surgery include male sex (4:1) and age in the 5th or 6th decade of life. Other factors may include difficult percutaneous lead placement, laminotomy approach to lead placement, and revision of previously placed leads. The need to perform surgical instrumentation and to create a bony insult dramatically increases the risk of a significant bleed.
The diagnosis of epidural hematoma is assisted by clinical suspicion, physical examination, and history, but the confirmatory diagnosis is made by CT scan. MRI can be obtained once the leads are removed. Early neurosurgical consultation is suggested if epidural hematoma is on the differential.
15.1.2 Epidural Abscess
Another major complication of the neuraxis associated with SCS is epidural abscess, one of the infectious risks of implanting devices in the body. (Other risks include incisional infection, cellulitis, meningitis, and discitis.) The risks of a serious infection appear to be less than 1 in 1000. Epidural abscess may present with severe pain in the area of the lead implant. This pain may be associated with fever, usually over 101 °F. Radicular pain may develop if the abscess extends to the canal or compresses the cord. Risk factors for abscess include immunocompromised state, history of chronic skin infections, history of methicillin-resistant Staphylococcus aureus (MRSA) infection or colonization, chronic diseases such as poorly controlled diabetes mellitus, or local infection at the surgery site. Abscess is diagnosed by clinical suspicion, history, and physical examination, and is confirmed by CT scan. MRI may be performed once the device is explanted.
15.1.3 Other Neurologic Injury
Neurologic injuries of the spinal cord or nerve roots are other potential risks of SCS. Injury may occur by needle trauma, lead placement or removal, or surgical manipulation during paddle lead placement. Neurologic injury is more common with paddle placement than with percutaneous cylindrical lead placement.
In many patients, the injury is associated with deep sedation or general anesthesia. In the immediate postprocedure period, the injury may be difficult to diagnose. CT scans may not show an abnormality, and MRI cannot be performed until the device is surgically removed. An electromyogram and nerve conduction study may be helpful in determining the injury, but findings may not become abnormal for several days after the insult.
Less worrisome complications include inadvertent dural puncture with post–dural puncture headache, which has been reported in up to 11 % of cases, although that number appears much higher than clinical practice would suggest. This risk is increased by obesity, calcific ligaments, patient movement, and previous surgery at the level of needle entry. A paramedian approach with an angle of less than 40° appears to lower the risk of complications.
Spinal cord stenosis can develop over time in the vicinity of an implanted lead; it may result in new radicular symptoms and can progress to myelopathy over time. This problem requires revision, decompression, or lead removal.
15.2 Complications Outside the Neuraxis
Reported incidences of wound infections involving the generator, tunneled area, or lead incision site have ranged from 0 to 4.5 % of patients. This problem is diagnosed by pain, swelling, rubor, and drainage of purulent material (Figs. 15.1 and 15.2). An elevated white blood cell count, sedimentation rate, or C-reactive protein should create concern regarding the infectious status of the implant. Other causes of infection should also be considered.
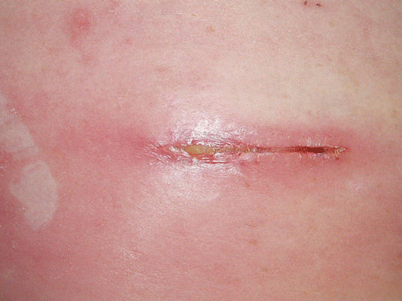
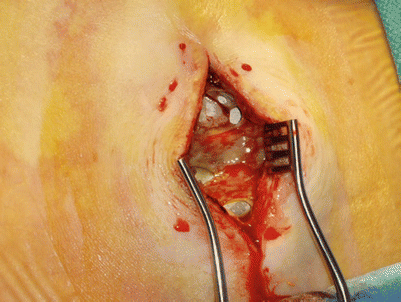

Fig. 15.1
Postoperative cellulitis with early dehiscence

Fig. 15.2
Gross infection present at generator site
Some patients may develop a swollen, irritated wound that is not associated with infection. This complication, termed a seroma, is caused by a buildup of serosanguinous fluid, and occurs with a frequency of 0.9–5.8 %. Seroma is diagnosed by lack of fever and a normal white blood count. If the diagnosis cannot be determined, incision and drainage with cultures may be required to make a conclusive diagnosis. In most cases, seroma can be treated without device removal. Careful dissection and attention to minimizing tissue trauma may reduce the risk of this complication. Compression with an abdominal binder, if appropriate, reduces the chance of seroma development.
Bleeding can occur at the generator site or lead incision. The result can be hematoma requiring drainage, or wound dehiscence. The best treatment is prevention, which consists of thoughtful tissue dissection, pressure to the area of bleeding, suturing of arterial bleeding, coagulation of ongoing small vessel hemorrhage, and careful inspection of the wound prior to closure.
Pain at the generator site may occur secondary to neuroma, tissue irritation, or bony contact with a rib or pelvic bones. Treatment can include topical local anesthetic patches, wound injection, or surgical revision.
15.3 Device-Related Complications
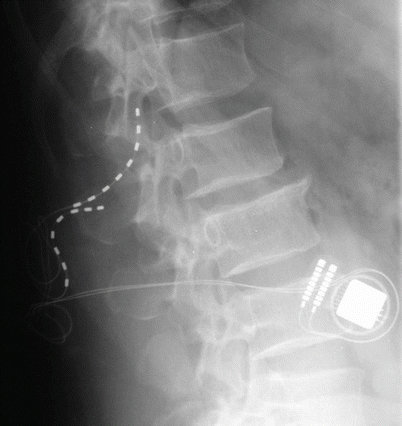
The most commonly reported complication of SCS devices is loss of paresthesia capture over time, which can result from lead migration, the patient’s development of tolerance to stimulation, or fibrosis below the lead, which increases impedance. Many of these problems can now be overcome by changes to the device. If reprogramming the system does not resolve the situation, plain films of the leads may be helpful in diagnosing migration. Eventual treatment may require lead revision or conversion to a percutaneous or traditional surgical paddle lead.
Lead migration is another complication that can lead to system failure (Fig. 15.3). This problem plagues both percutaneous and paddle systems. The authors have experienced less than 1 % migration based on x-ray evaluation, and recent studies have shown the number to be less than 11–13 % in most evaluations. The problem is diagnosed with loss of therapeutic stimulation not overcome by reprogramming, dramatic change in location or characteristics of the stimulation, and by comparison of anterior-posterior and lateral films with the original implant films. Treatment commonly requires surgical lead revision. Careful attention to anchoring to the lumbodorsal fascia may reduce the risk of this complication but cannot prevent it entirely.