Chapter 1 Complications of Spinal Cord Stimulation
 Appropriate Accreditation Council for Graduate Medical Education mentored subspecialty training in interventional pain management is vital to ensure patient-centered care.
Appropriate Accreditation Council for Graduate Medical Education mentored subspecialty training in interventional pain management is vital to ensure patient-centered care. Accurate and expertly placed needles in appropriately selected patients are essential to ensure accurate diagnosis and successful treatment.
Accurate and expertly placed needles in appropriately selected patients are essential to ensure accurate diagnosis and successful treatment. Complications associated with spinal cord stimulation (SCS) rarely impact patient morbidity or mortality.
Complications associated with spinal cord stimulation (SCS) rarely impact patient morbidity or mortality. Approximately 30% to 40% of patients undergoing SCS will have a complication, and nearly 80% of them require a revision.
Approximately 30% to 40% of patients undergoing SCS will have a complication, and nearly 80% of them require a revision. Biologic complications commonly occur within 3 months of implant, although infections can rarely present much later. Technical complications typically occur within 2 years of implant.
Biologic complications commonly occur within 3 months of implant, although infections can rarely present much later. Technical complications typically occur within 2 years of implant. Common technical complications include lead migration and lead fracture and (less commonly) battery malfunction.
Common technical complications include lead migration and lead fracture and (less commonly) battery malfunction.Introduction
Neurosurgical treatments for pain have historically fallen under the headings of anatomic, ablative, or augmentative, and before the gate control theory was proposed by Melzack and Wall,1 little attention was directed toward augmentative therapies. Spinal cord stimulation (SCS) has become one of the most useful interventional therapies to treat neuropathic and ischemic pain since its introduction as an intrathecal device by Shealy et al2 in 1967.
Current estimates suggest that more than 27,000 spinal cord stimulator implants were performed in 2007 alone in the United States,3 and this number will likely increase as SCS is used earlier in treatment algorithms for neuropathic pain,4 supported by cost effectiveness and successful outcomes.5
One can appreciate that increased use of the technique also increases the incidence and prevalence of associated complications, which range from the mundane to the devastating. Recent reviews place the overall incidence of complications associated with spinal cord stimulation to be approximately between 14% and 43%.6 Cameron7 performed an extensive review of the literature extending over 3679 patients, concluding an overall mean complication rate with SCS of 36%. Moreover, Kumar et al8 reported that complications requiring revisions were approximately 25% to 33% and approximately 85% of reoperation patients were satisfied with the results.9 Furthermore, Rosenow et al10 retrospectively determined that in 289 patients who underwent spinal cord stimulator implants, 46% required revision, and of those, almost half required more than one.
As with any treatment plan, complication avoidance begins with patient selection. Patients with local infection near the injection site, coagulopathy, allergy to injectate, or comorbidities or conditions that prevent fluoroscopic needle guidance or consent should be avoided. Patient selection regarding psychometric testing deserves special mention. It is well established that concurrent psychiatric illness reduces interventional treatment success rates11 and that approximately 20% to 45% of pain patients have accompanying psychopathology.12 Therefore it is essential that appropriate measures be taken to diagnose, treat, or exclude unsuitable candidates. Instruments described to aid in identifying the presence of clinically significant psychopathology include the Symptom Checklist 90 (SCL-90-R) and the Minnesota Multiphasic Personality Inventory (MMPI-2). Poor treatment outcome was identified in patients with presurgical somatization, depression, anxiety, and poor coping.13
It is also crucial to appropriately use the spinal cord stimulator for the appropriate indications (neuropathic pain states and ischemic pain), as approved by the Food and Drug Administration. Disease states that yield themselves to successful treatment with SCS include postherpetic neuralgia, intercostal neuralgia, postlaminectomy pain, complex regional pain syndrome, phantom limb pain, angina, painful peripheral neuropathy, focal peripheral neuropathies, ischemic limb pain, and radiculitis and radiculopathy.14
A clear understanding of spinal anatomy, utilization of image guidance, and proper surgical technique are vital to ensure both quality treatment and reduced patient morbidity and mortality.15 Furthermore, it should be understood that appropriate training within Accreditation Council for Graduate Medical Education accredited programs and mentorship is pivotal to ensure treatment success and limit iatrogenic morbidity; interventional hobbyists and weekend crusaders only serve to undermine the accessibility of this valuable therapy to patients.
Spinal cord stimulator complications can be broadly divided into those that are technical or those that are biologic or surgical in nature. The consequences of these complications can range from the mundane to the devastating. Most complications of SCS do not impact patient mortality,7 unlike that for intrathecal drug delivery, as recently reported by Coffey et al.16 Although the range of various complications varies from 0% to 81% across studies,17 the frequencies of the complications in the tables in this chapter are presented as the means. Refer to Table 1-1 for reported rates of complications, revisions, and explants associated with SCS.
Table 1-1 Reported Complications of Spinal Cord Stimulation
| Complication | Frequency |
|---|---|
| Overall | 14%–43%6,17 |
| Complication requiring revision | 25%–33%,8 23%17 |
| Complication requiring explant | 11%17 |
Selected Complications
Technical Complications
Innately, the restrictions current technology places on the spinal cord stimulator device plays a role in the associated complications. Advances in system technology have largely reduced many early complications related to device failure, including loss of stimulation. Van Buyten et al18 described such a relationship, commenting in a 5-year prospective study of 84 patients with 85 spinal cord stimulator systems, with overall 54% of patients reporting greater than 50% pain relief at 5 years. Satisfaction and employment of the patient programmer were at least 95% after stimulator therapy began (Table 1-2).
Table 1-2 Technical Complications Reported for Spinal Cord Stimulation
| Complication | Frequency |
|---|---|
| Lead migration (loss of stimulation) requiring revision | 11%,21 13.2%22 |
| Lead integrity violation requiring revision | 6%,21 9%22 |
| Loose connection | 0.4%7 |
| Hardware malfunction, equipment failure | 2.9%,7 6.5%17 |
| Battery failure | 1.6%7 |
| Central nervous system electrical injury from aberrant stimulator activation | Case report25 |
| Misplacement of epidural lead | Case report24 |
Furthermore, stimulation, regardless of the choice of the numerous mechanisms proposed,14 depends on the integrity and characteristics of the “circuit created.” Spinal cord stimulator contact to target structure length varies with patient position, as described by Abejon and Feler,19 and predictively, so too do the circuit created and the resultant therapeutic stimulation. Recumbence reduced impedance and energy requirement (measured in thoracic and cervical implanted leads), and therefore stimulation was perceived to be “stronger,” although the difference was not statistically significant. Clinical implications longitudinally require further study.
Lead Migration
The most common complication of SCS is lead migration7,14,17,20 and loss of therapeutic stimulation. Cameron et al7 reported that 27.2% of spinal cord stimulator cases were technical in nature, and of those, 87% were related to the lead. Estimates place migration incidence to be between 11% and 13%, with a greater propensity for migration in the cervical spine,8 likely related to greater mobility, compared with the lumbar spine.
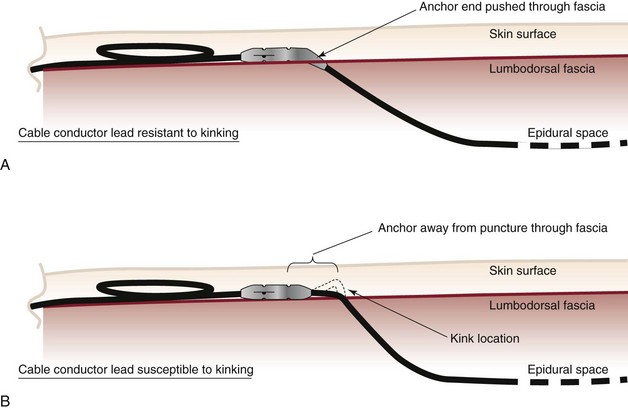
Understandably, migration occurs when directional forces and tensile load on the electrode exceed the stabilizing forces of the anchor. These forces depend on lead-spanning mobile areas, trajectory into the epidural space, anchor orientation, the type of tissue the anchor is sutured to, suture technique, and the site of battery placement. It has been suggested that percutaneous electrodes are more prone to migration than their laminectomy counterparts and that battery location in the abdomen may be associated with less lead migration than in the gluteal region, however work by Henderson and Kumar suggest otherwise. Those studies have suggested that the closer the implanted pulse generator (IPG) is to the lead insertion site, the lower the risk of lead movement. IPG placement choices include the buttocks, midflank, abdominal wall, or infraclavicular regions.7 Rosenow et al,10 however, in a study of 289 patients, reported that migration was slightly higher in the surgical laminectomy lead.
Mitigating migration involves reducing iatrogenic cofounders. These include the angle of spinal cord lead entry into the epidural space, stress loops, battery site choice, and anchor or suturing technique. Bedder and Bedder15 detail basic surgical technique and nomenclature that will not be further discussed here. Kumar et al8 suggest that proper anchoring and placement of the tip of the anchor into the supraspinal ligament might reduce anchor failure. Strain relief loops of approximately 1 inch in diameter, both near the anchor and at the generator site, may reduce tensile load (Fig. 1-1).
Lead Fracture
Lead fracture occurs approximately 5% to 9%,21–23 with contradictory reports regarding fracture rates with percutaneous versus laminectomy leads. Kumar et al23 described percutaneous lead fracture more often than laminectomy leads; in contradiction, Rosenow et al10 reported more lead fracture with laminectomy. Clearly, lead fracture depends on lead tensile strength, the friction coefficient of the internal wires composing the lead, and a vector of force that challenges the lead integrity. Iatrogenic ways to lessen lead fracture are similar to strategies used to reduce lead migration and include appropriate anchoring, using stress loops, placement of the IPD in the abdomen,8 and avoidance of tunneling around mobile structures (i.e., joints).
Hardware Malfunction and Battery Failure
Exclusive to lead migration of fracture, other hardware complications occur with a frequency of 2.9% and include battery failure (exclusive to predicted nonrechargeable battery depletion) with a frequency 1.6% per Cameron et al.7 Turner et al17 described equipment failure rates of 6.5%.
Misplacement of Epidural Lead
Therapeutic stimulation requires epidural placement of the spinal cord stimulator lead. Intrathecal and subdural placement have been reported.24 Troubleshooting of lead placement centers on fluoroscopic guidance and impedance testing. Anterior epidural lead placement is suggested by muscle contraction of adjacent or distal spinal nerves and painful stimulation within the appropriate expected stimulation parameters. Intrathecal lead placement is suggested by very low-impedance testing, and the hallmark of subdural stimulation is segmental, low-amplitude stimulation that is often uncomfortable to the patient. A case report of intramedullary lead placement is described in the next section.
Aberrant Stimulation
Aberrant activation of stimulation rarely occurs, although an infamous case report is often sited. Eisenberg and Waisbrod25 described a patient who had a cervical spinal cord stimulator that was activated by an antitheft device. Six months after implantation, upon entering a store, the patient felt an electrical shock in the back of his skull and fell unconscious. He awoke in the emergency department with confusion, ataxia, upper extremity intention tremor, weakness, and dysarthric speech. Initial computed tomography (CT) scan and electroencephalogram were normal, although 6 months later, with continued symptoms, follow-up CT demonstrated a left basal ganglia infarct. The authors postulate that the anti-theft device triggered a sudden bolus of current, causing damage that contributed to the injury.
Kumar et al8 reported that whereas device or technical complications typically occur within 2 years of implant, biologic complications of SCS typically occur within 3 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree