Complications in Pain Medicine
James P. Rathmell
Complications arise in the course of any type of medical treatment, and the common interventions used to treat acute and chronic pain are no exception. The best approach to preventing adverse outcomes during the majority of these treatments is to have detailed knowledge of the patient’s medical condition and of the pharmacology/physiology of the intervention. Also required are a detailed knowledge of the anatomy of the adjacent structures that lie near the target site for each intended treatment and a clear understanding of how the technique has been devised to minimize the risk of harm to these structures. Many pain procedures are now best carried out with the use of radiographic guidance, and the widespread availability of fluoroscopy has increased both the precision and safety of many techniques. When complications do arise, prompt recognition and treatment can often prevent serious sequelae. This chapter reviews complications associated with many of the treatments used in the practice of pain medicine.
Complications Associated with Epidural, Facet Joint, and Sacroiliac Injection
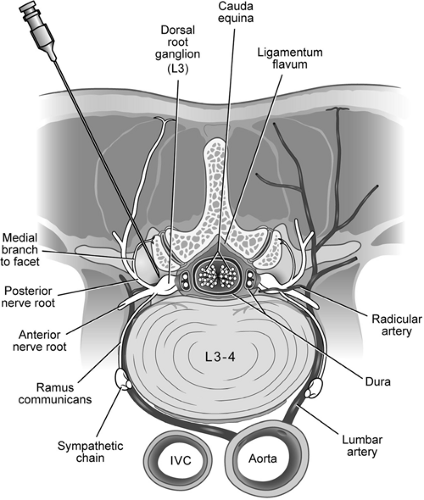
Direct injection of local anesthetic with or without long-acting corticosteroid suspensions has been used in the diagnosis and treatment of pain arising from spinal structures. Among the most common applications is the injection of corticosteroid into the epidural space in efforts to reduce the pain associated with acute lumbar disc herniation and spinal stenosis (1,2). In those with acute radicular pain caused by herniation of an intervertebral disc, epidural steroid injection appears to speed the resolution of acute pain. The degenerative cascade that leads to loss of intervertebral disc height with ageing can cause increased stress on the zygapophyseal (facet) joints, and these facet joints are well recognized as a source of chronic axial back pain. Injection of the local anesthetic and corticosteroid within the facet joints has been used with limited success to treat axial back pain (3). Injection of local anesthetic along the course of the medial branch nerve to the facet joints is often used as a diagnostic test before proceeding with radiofrequency ablation. The sacroiliac joint (SIJ) is a less common cause for axial low back pain, and injection of local anesthetic into the joint has been used as a diagnostic test for SIJ-related pain, whereas injection of corticosteroid has been used with limited success to afford longer-term pain resolution (4).
Few reports of serious complications are associated with epidural, facet joint, and sacroiliac joint injection of corticosteroids. However, it is difficult to estimate the actual incidence of complications with any certainty, as no mandatory reporting system exists for such adverse events in the United States. The American Society of Anesthesiologists (ASA) maintains an ongoing surveillance of malpractice claims that have been settled through the ASA Closed Claims study, and a recent report stemming from this project detailed 114 complications associated with chronic pain treatment (Table 50-1) (5). In addition, there remain significant concerns regarding the neurotoxic potential of the available corticosteroid preparations (6). Neurotoxicity, neurologic injury, and the pharmacologic effects of corticosteroids have all been reported as complications following epidural, facet joint, and sacroiliac corticosteroid injections.
Neurotoxicity
The intrathecal injection of neurotoxic substances can result in inflammation of the meninges with or without direct neural injury in the form of arachnoiditis or cauda equina syndrome. Arachnoiditis is an inflammatory condition of the meninges that can extend to the underlying neural structures. This inflammation usually follows significant spinal infection, often following tertiary syphilis or advanced tuberculosis (7). However, arachnoiditis can also arise following the intrathecal injection of radiographic contrast or following surgical breach of the spinal canal during spinal surgery. Cauda equina syndrome is a broad descriptive term that refers to neurologic signs and symptoms that arise from direct compression of the cauda equina. The syndrome is characterized by bilateral sciatica, saddle hyperesthesia, lower extremity weakness; and bowel, bladder, and sexual dysfunction. Although cauda equina syndrome is most often seen in association with a compressive lesion (e.g., epidural extension of a metastatic tumor or a large central disc herniation), similar symptoms can also arise in association with severe arachnoiditis. The typical signs and symptoms of arachnoiditis are shown in Table 50-2 (see also Fig. 50-30).
Concern regarding the potential for neurotoxicity associated with epidural steroid injections stems from reports of arachnoiditis that arose during the course of repeated intrathecal injections administered for the treatment of multiple sclerosis (8,9,10). Two cases of arachnoiditis were reported among a series of 23 patients receiving repeated injections of intrathecal methylprednisolone acetate (MPA), and concern was raised about the drug vehicle, polyethylene glycol, as the possible causative agent (8). Only one case of documented arachnoiditis has been reported following epidural steroid injection for sciatica due to lumbar disc disease complicated by a traumatic tap; the symptoms resolved following subsequent discectomy (11).
Table 50-1 Primary outcome for claims related to epidural steroid injections | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Aseptic meningitis has also been reported following intrathecal injection of MPA (12,13). This condition can arise after the intrathecal injection of nearly any substance, including normal saline (14). Aseptic meningitis is generally a benign and self-limited condition that produces signs of neurologic irritation, including burning pain in the legs, headache, meningismus, and, in severe cases, seizures. Fever and nausea are often reported. Cerebrospinal fluid (CSF) examination reveals pleocytosis, elevated protein, and decreased glucose. Several cases of aseptic meningitis have been reported after intrathecal corticosteroid injection (12,15,16) and one case has been reported after epidural (17) corticosteroid injection. In at least one case (17), the symptoms were severe and prolonged, resolving over a period of more than 3 weeks.
Limited evidence suggests that any component of the available long-acting corticosteroid preparations may be neurotoxic. Following the appearance of arachnoiditis during the intrathecal injection of MPA, propylene glycol was suggested as the offending agent (6). The polyethylene glycol preparation used in steroid suspensions has a molecular weight of 3,350 and is present in concentrations of 2.8% to 3%. However, propylene glycol has no acute effects on either sheathed or unsheathed neurons in concentrations up to 10%, with mild slowing appearing at 20% to 30%, and complete abolishment of conduction at 40% (18). This effect was reversible following washout in both sheathed and unsheathed nerves. Benzyl alcohol 0.9% is present as a preservative in several steroid suspension preparations, including multidose vials of Depo-Medrol brand MPA (Pharmacia & Upjohn, Kalamazoo, MI) and Aristocort Intralesional brand triamcinolone diacetate (American Cyanamid, Madison, NJ). Mild, transient inflammatory changes appear in the nerve roots, cord root entry zone, and meninges after epidural injections of triamcinolone diacetate plus lidocaine in cats (19); however, no discernible effects on neurologic function or histology were seen in a separate study examining repeated intrathecal injections of triamcinolone diacetate (20). Direct intrathecal injection of up to 9% benzyl alcohol (10 times the concentration used as a preservative) in dogs produced transient neurologic dysfunction related to the local anesthetic effect of this agent without the appearance of any discernible long-term sequelae or histologic changes (21).
Table 50-2 Symptoms of adhesive arachnoiditis | |
|---|---|
|
Much public controversy surrounded the risk of arachnoiditis following epidural MPA administration in Australia during the 1990s. This led some practitioners to adopt the use of Celestone Chronodose (betamethasone 5.7 mg, as betamethasone sodium phosphate 3.9 mg [in solution] and betamethasone acetate 3 mg/mL [in suspension] in an aqueous vehicle; Schering-Plough, Kenilworth, NJ) for epidural injection. This product contains sodium phosphate monobasic, disodium edetate, benzalkonium chloride, and water. However, a study in sheep demonstrated arachnoiditis in animals receiving 2 mL or more of this preparation intrathecally (22). More recently, practitioners have moved to the use of the potent, soluble steroid dexamethasone for epidural use; a recent study in rodents found no evidence of arachnoiditis or neural injury following intrathecal administration of this agent (23).
It is not clear that a single intrathecal injection of any of the available corticosteroid preparations will cause any harm. Indeed, a recent study of repeated intrathecal administration of MPA for postherpetic neuralgia failed to find any evidence of either aseptic meningitis or arachnoiditis among 89 patients treated with four 60-mg injections (24). However, it is important for practitioners to realize that, despite their widespread use for epidural injection, the U.S. Food and Drug Administration nor any other regulatory agency labels these steroid preparations for epidural use. The concern regarding arachnoiditis appears to be limited to intrathecal administration (e.g., following inadvertent dural puncture in the course of attempted epidural placement); large case series and randomized trials of epidural injections of long-acting particulate steroids are devoid of reports of arachnoiditis. Thus, the most prudent approach is to use all means available to avoid intrathecal injection of corticosteroid. A local anesthetic test dose administered prior to steroid injection can effectively rule out intrathecal placement. Use of radiographic guidance and injection of a small amount of radiographic contrast can also be used to accurately confirm epidural localization of the injectate. In those patients in whom a dural puncture occurs during needle placement, it is prudent to consider abandoning the procedure; placement of the corticosteroid at an adjacent interspace will reduce, but not completely eliminate the chance of the steroid reaching the intrathecal space.
Neurologic Injury
Direct injury to the spinal nerves or the spinal cord itself can occur during needle placement for epidural injection. The most common form of nerve injury is persistent paresthesia. More severe injury to the spinal cord can occur when the advancing needle enters the substance of the spinal cord. Surprisingly, needle penetration into the cord is not in itself always catastrophic and may even occur without the patient reporting symptoms (25). More significant injury occurs if bleeding into the spinal cord occurs or if injectate of any kind is placed through the needle into the substance of the spinal cord (26,27). Neural injury occurred in 14 patients following epidural steroid injection reported in the Closed Claims Study (5). Six of these resulted in paraplegia, one in quadriplegia. Another mechanism of injury is injection of steroid suspension into a radicular artery, with embolization of end arteries in the spinal cord or cerebellum; this complication will be discussed in detail in the section later in this chapter on transforaminal injection of steroids.
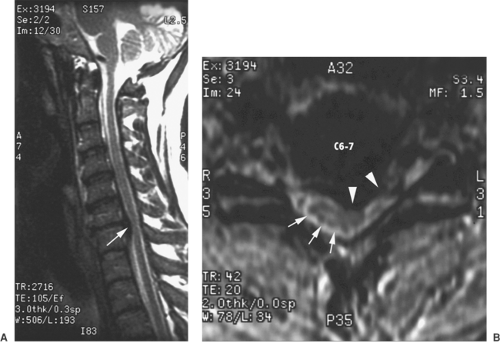 Figure 50-1. Cervical magnetic resonance imaging (MRI) of large disc herniation causing effacement of the epidural fat and cerebrospinal fluid (CSF) surrounding the spinal cord. Field et al. (29) reported three cases of transient neurologic injury that followed otherwise uneventful cervical epidural steroid injections in awake patients. All three patients had large disc herniations causing effacement of the epidural fat and spinal fluid surrounding the spinal cord at the level of injection. A: Midline sagittal, T2-weighted MRI showing large disc herniation at the C6–7 level (arrow) that effaces the epidural fat and CSF signal both anterior and posterior to the spinal cord. B: Axial, T1-weighted MRI, showing a large central and left-sided disc herniation (arrow heads) displacing the spinal cord (arrows) to the right posterolateral limits of the spinal canal. Reproduced from Field J, Rathmell JP, Stephenson JH, Katz NP. Neuropathic pain following cervical epidural steroid injection. Anesthesiology 2000;93:885–888, with permission. |
The risk of direct injury to the spinal cord is greatest when epidural injection is carried out at the high lumbar, thoracic, or cervical levels, where the spinal cord lies directly anterior to the path of the advancing needle. Two cases of spinal cord injury following cervical epidural steroid injections conducted with fluoroscopic guidance were reported (28). The details of use of fluoroscopy were not given, and it is not clear if the final needle position was confirmed using both anteroposterior (AP) and lateral images and/or with use of radiographic contrast. In both cases, the patients received sedation with midazolam and propofol. The authors postulate that these patients failed to report any symptoms associated with needle contact with the cord because of the level of sedation. Both patients developed persistent upper extremity pain and lower extremity paresthesias. A more recent report details three cases of transient neurologic injury that followed otherwise uneventful cervical epidural steroid injections in awake patients (29). All three patients had large disc herniations that caused effacement of the epidural fat and spinal fluid surrounding the spinal cord at the level of injection (Fig. 50-1). The authors hypothesized that direct injury to the spinal cord or dorsal nerve root could occur even without dural puncture when narrowing or obliteration of the epidural space, caused by a large disc herniation, displaces the spinal cord posteriorly.
The use of fluoroscopy offers some protection against neural injury. The position of the needle tip in the AP view can be kept midline as it is advanced, eliminating the risk of lateral deviation and injury to the nerve roots or the spinal nerve. Although the primary means of detecting penetration of the epidural space remains the loss of resistance to injection as the needle is advanced, a lateral view on fluoroscopy can be used to assure that the needle tip is at the level of the posterior border of the bony spinal canal. Once the tip of the needle appears to be in good positron in both AP and lateral views, injection of a small volume of radiographic contrast can be used to assure that the injectate is within the epidural space. Most reports describe the immediate onset of severe pain in one or both lower extremities reported by awake patients receiving epidural injections who went on to develop spinal cord injuries (26,27,29). Thus, minimizing sedation is an important measure—the patient should be alert enough to respond to paresthesias induced by needle contact with neural structures. In the majority of cases, probably little more than supportive care can be offered to those patients who do suffer neural injury in the course of epidural injection. Immediate neurosurgical consultation and diagnostic imaging is warranted in those cases in which injury to the spinal cord is suspected; consideration should be given to administration of a course of high-dose intravenous (IV) corticosteroids,
as this approach has proven beneficial in reducing neuronal injury following traumatic spinal cord injury (30).
as this approach has proven beneficial in reducing neuronal injury following traumatic spinal cord injury (30).
Pharmacologic Effects of Corticosteroids
The administration of exogenous corticosteroids can lead to both hypercortisolism and suppression of the adrenal cortex’s normal production of endogenous glucocorticoids. Cushing’s syndrome occurs as the result of excessive endogenous cortisol production by the adrenal cortex and results in a characteristic pattern of obesity associated with hypertension. Prolonged administration of exogenous glucocorticoids can result in similar manifestations and is termed cushingoid syndrome. The long-acting corticosteroid preparations used for epidural steroid injection slowly release the active steroid over 1 to 3 weeks. Fluid retention and weight gain, increased blood pressure, and congestive heart failure have been reported after epidural steroid injections (31,32), and may be more likely in those with a history of previous congestive heart failure or chronic diuretic use. Cushingoid side effects have been reported even after a single epidural administration of corticosteroid (33).
Epidural administration of long-acting corticosteroid preparations leads to prompt, marked, and prolonged suppression of serum cortisol levels. In 12 patients who received 80 mg of epidural methylprednisolone acetate, adrenocorticotropic hormone (ACTH) and plasma cortisol levels were depressed from 1 to 21 days after treatment, and the ability of exogenous ACTH to raise plasma cortisol levels was reduced over the same interval (34). Three epidural injections of 80 mg of triamcinolone acetate given at weekly intervals also reduced ACTH and serum cortisol levels, starting within 45 minutes of the first injection; levels were nearly normal 30 days following the last injection (35). Steroid-induced myopathy, characterized by progressive proximal muscle weakness, increased serum creatinine kinase levels, and a myopathic electromyelogram (EMG) and muscle biopsy specimen has been reported following a single epidural dose of triamcinolone (36). Severe cases of cushingoid syndrome and steroid myopathy are rare, and these have been reported after a single epidural injection of corticosteroid, thus it is unlikely that these complications can be completely avoided. However, the use of systemic corticosteroids in any form is not without risk, thus making it essential to judge the response from each treatment before administering additional corticosteroid. No specific treatment is available for the adrenal suppression that follows epidural injection of corticosteroid; however, it seems prudent to consider coverage with an additional dose of exogenous steroid in those undergoing major surgery in the weeks following epidural steroid injection.
Glucocorticoid administration reduces the effect of insulin and results in increased blood glucose levels and insulin requirements in diabetics for 48 to 72 hours. A single caudal epidural injection of triamcinolone acetonide resulted in an increase in serum insulin levels and a suppression of serum glucose response to insulin within 24 hours, and a return to normal after 1 week (37). There is little published information about the effects of epidural injection of steroids on glucose control in diabetic patients. Glucose levels in diabetic patients should be monitored closely during the week following administration of any type of long-acting steroid. Patients must be informed that adjustment of insulin dose may be required. Brittle diabetics should consult their internist or endocrinologist prior to initiating steroid treatment.
Although rare, allergic reaction to systemic administration of corticosteroid has been documented (38,39). Signs and symptoms of a delayed allergic reaction appeared in one patient a week after epidural injection of triamcinolone diacetate, and subsequent skin testing resulted in recurrence of symptoms (40).
Bleeding Complications
Similar to single-shot epidural placement for surgical anesthesia, epidural injection for pain treatment carries the risk of intraspinal bleeding. Significant bleeding within the epidural space can cause compression of neural elements and potentially result in paraplegia or quadriplegia. Both epidural (41) and subdural (42) hematomas have been reported following epidural steroid injections in patients without any apparent coagulopathy. The patient with the epidural hematoma regained normal neurologic function following emergent surgical decompression. The patient with the subdural hematoma initially developed quadriplegia; although neurologic function recovered after surgery, the patient subsequently developed meningitis and died. A case of quadriplegia following cervical epidural steroid injection in a patient receiving clopidogrel and diclofenac has also been reported; this patient regained upper extremity function after surgical decompression (43). In the Closed Claims Study (5), two epidural hematomas occurred following epidural steroid injections; both patients had been receiving anticoagulants.
The risks and considerations regarding neuraxial blockade in patients receiving anticoagulation are similar in those receiving the injections for treatment of pain to those who are receiving epidural anesthesia or perioperative epidural analgesia (44). Epidural injection of steroids should be avoided in patients receiving systemic anticoagulants (e.g., Coumadin or heparin) or potent antiplatelet agents (e.g., clopidogrel or ticlopidine). However, nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, do not appear to increase the risk of epidural hematoma formation associated with epidural injection. In a series of 1,035 patients who received a total of 1,214 epidural steroid injections, no bleeding complications were seen; one-third of these patients had been taking NSAIDs at the time of treatment (134 on aspirin, 249 on other NSAIDs, 34 on multiple NSAIDs) (45).
Infectious Complications
Injection therapy for pain treatment carries a small risk of both superficial and deep infection, including neuraxial infection such as epidural abscess (46) (see also Fig. 50-22). Both superficial and deep infections have been reported after injection therapies for pain including epidural steroid injections (47,48,49), facet injections (50), and trigger point injections (51). It is not possible to discern the actual incidence of infection from the available published data.
The most worrisome and potentially devastating infectious complication is epidural abscess. Abscess formation within the epidural space can occur without injection or instrumentation of the spinal canal. In a series of 46 cases of spontaneous epidural abscess, 46% occurred in diabetic patients (52). Common presenting symptoms included paralysis (80%), localized spinal pain (89%), radicular pain (57%), and chills and fever (67%). The erythrocyte sedimentation rate (ESR) was always elevated. Staphylococcus aureus was the most common organism isolated. A recent review detailed 14 cases of epidural abscess following epidural steroid injection that have been reported in the literature (53). Patient characteristics and outcomes for those cases and one additional reported case (48)
are shown in Table 50-3. Infection was listed in the Closed Claims Study (5) as a cause for litigation in 24 cases involving epidural steroid injections. Twelve cases of meningitis and three cases of osteomyelitis occurred. Seven cases of epidural abscess were noted, six requiring surgical decompression and one resulting in permanent lower extremity motor dysfunction. In one claim, both meningitis and epidural abscess occurred, and in one a combination of meningitis, abscess, and osteomyelitis.
are shown in Table 50-3. Infection was listed in the Closed Claims Study (5) as a cause for litigation in 24 cases involving epidural steroid injections. Twelve cases of meningitis and three cases of osteomyelitis occurred. Seven cases of epidural abscess were noted, six requiring surgical decompression and one resulting in permanent lower extremity motor dysfunction. In one claim, both meningitis and epidural abscess occurred, and in one a combination of meningitis, abscess, and osteomyelitis.
Table 50-3 Data regarding cases of epidural abscess following epidural steroid injections | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Similar to epidural infections, the majority of cases of septic arthritis of the facet and sacroiliac joints occur in the absence of injection or instrumentation. Systematic reviews have reported 27 cases of facet joint infection (54) and 166 cases of bacterial sacroiliitis (55). Following intraarticular facet injection, septic arthritis in the facet joints can extend to involve the paraspinous muscles (56) and the epidural space (57).
No well-tested guidelines for the prevention of infection during injection treatment for chronic pain are available. Considerations regarding sterile technique and use of disinfectant solutions are similar to those recommended for single-shot regional anesthetic techniques performed in the perioperative period (58,59). Most experts recommend the use of an iodine-based skin preparation solution, routine use of sterile drapes and gloves, and strong consideration of routine use of face masks and hats. Routine use of preprocedure antibiotics does not appear to be warranted in the majority of single-shot spinal and perispinal injections (46). Pain practitioners should establish written postprocedural guidelines for their patients that include a clear description of the signs and symptoms of evolving infection and a clear process for contacting pain clinic personnel to report the appearance of any worrisome signs or symptoms (46). Although some isolated paraspinous infections have been treated with needle aspiration, most will require open surgical incision and drainage along with the administration of systemic antibiotics.
Complications Associated with Transforaminal Injections
Transforaminal injections are those delivered onto a spinal nerve within the intervertebral foramen. The rationale for injecting steroids is that they suppress inflammation of the nerve, which, in many instances, is believed to be the basis for radicular pain (60,61). The rationale for using a transforaminal route of injection rather than an interlaminar route is that the injectate is delivered directly onto the target nerve. This ensures that the medication reaches the site of the suspected pathology in maximum concentration. When used for diagnostic purposes, transforaminal blocks can be used to pinpoint the particular spinal nerve responsible for radicular pain when imaging studies are ambiguous; the drug injected is a local anesthetic agent. When used as a therapeutic intervention, a combination of a local anesthetic and a corticosteroid preparation is injected. Most practitioners use lidocaine (in concentrations of 0.5%, 1%, or 2%) but some use small volumes of bupivacaine (0.5%). The steroid preparation depends on practitioner preference, and available choices include betamethasone, dexamethasone, and methylprednisolone. Numerous reports document complications associated with both the cervical and lumbar transforaminal injection of steroids.
Cervical Transforaminal Injections
The most concerning risk of transforaminal injection involves unintentional vascular injection of the steroid solution. The incidence of intravascular injection was 19% in a series of 504 cervical transforaminal injections (62). Although the authors do not differentiate intra-arterial and IV injections, the observed vascular injections seem to have been IV, and no adverse outcomes occurred. Intravenous injection is an innocuous event during transforaminal injection; particulate steroid injected intravenously will simply be carried away from the site of inflammation, thus reducing any local anti-inflammatory effect. In contrast, intra-arterial injection is far less common, but the effects may lead to catastrophic neurologic injury (63).
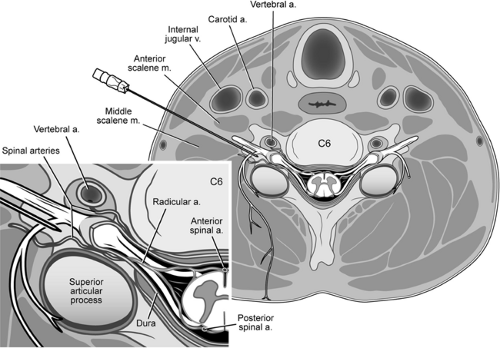
In the cervical spine, the vertebral artery, the ascending cervical artery, and the deep cervical artery each furnish spinal branches that enter the intervertebral foramina. These spinal branches supply the vertebral column but also give rise to radicular arteries that accompany the dorsal and ventral roots of the spinal nerves (Fig. 50-2). Not infrequently, anterior radicular arteries are of significant caliber and reinforce the anterior spinal artery. Such reinforcing arteries can occur at any cervical level, but appear to be more common at lower cervical levels (64). If particulate steroid is injected within a reinforcing radicular artery during transforaminal injection, infarction of the cervical spinal cord could ensue. The vertebral artery lies anterior to the cervical intervertebral foramina, and should not be encountered in a carefully executed transforaminal injection. However, radicular arterial branches arising from the vertebral artery can also join the arterial supply that reaches the anterior spinal artery; it is feasible that injectate placed within a radicular artery during transforaminal injection could reach the vertebral artery via retrograde flow through an arterial anastomosis (64). If particulate steroid reaches the vertebral artery during transforaminal injection, infarction of the posterior circulation of the brain, including the cerebellum, could ensue.
The first report of a complication attributed to cervical transforaminal injection of steroids described a patient who died from a spinal cord infarction (65). The location of the infarction implied that a radicular artery that reinforced the anterior spinal artery had been compromised, but no evidence was offered about the mechanism by which the artery had been compromised. Images of the placement of the needle were not published. A subsequent case report did describe a possible mechanism by which the spinal cord could be compromised
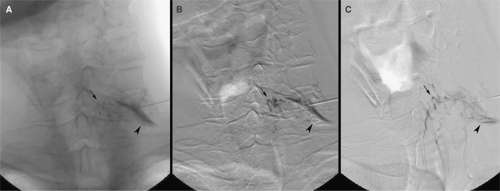
(66). During a C5–C6 transforaminal injection, the operator injected a test dose of contrast medium to ensure that the injectate properly dispersed along the course of the spinal nerve and its root sleeve. At that time, filling of a transversely running vessel that satisfied the features of a radicular artery was observed (Fig. 50-3). The procedure was terminated and the patient suffered no ill effects. The authors postulated that unintentional injection of an anterior radicular artery could occur during transforaminal injection. If particulate steroids were to be injected into a reinforcing artery, they could act as an embolus to infarct the spinal cord. This view was reiterated in a later review article (63), which described another case of radicular artery filling during cervical transforaminal injection. Further circumstantial evidence of this mechanism was provided in another case report (67). Upon injecting contrast medium, the operator found no evidence of intra-arterial injection during a C6–C7 transforaminal injection. He next injected local anesthetic. The patient developed neurologic features consistent with anesthetization of the anterior and anterolateral columns of the cervical spinal cord. The posterior columns were spared. These features indicate that injection was made into a radicular artery, which reinforced the anterior spinal artery. The procedure was terminated and the patient recovered within 20 minutes.
(66). During a C5–C6 transforaminal injection, the operator injected a test dose of contrast medium to ensure that the injectate properly dispersed along the course of the spinal nerve and its root sleeve. At that time, filling of a transversely running vessel that satisfied the features of a radicular artery was observed (Fig. 50-3). The procedure was terminated and the patient suffered no ill effects. The authors postulated that unintentional injection of an anterior radicular artery could occur during transforaminal injection. If particulate steroids were to be injected into a reinforcing artery, they could act as an embolus to infarct the spinal cord. This view was reiterated in a later review article (63), which described another case of radicular artery filling during cervical transforaminal injection. Further circumstantial evidence of this mechanism was provided in another case report (67). Upon injecting contrast medium, the operator found no evidence of intra-arterial injection during a C6–C7 transforaminal injection. He next injected local anesthetic. The patient developed neurologic features consistent with anesthetization of the anterior and anterolateral columns of the cervical spinal cord. The posterior columns were spared. These features indicate that injection was made into a radicular artery, which reinforced the anterior spinal artery. The procedure was terminated and the patient recovered within 20 minutes.
Several reports have now appeared implicating vertebral artery injection as the mechanism of injury during cervical transforaminal injection. After attempted C5–C6 and C4–C5 transforaminal injections, a patient developed bilateral blindness, and magnetic resonance imaging (MRI) revealed bilateral parenchymal enhancement of the occipital lobes (68). The clinical features and the imaging results implicated unintentional injection into the vertebral artery. The offending agent was not apparent, as only contrast medium and air were used during the procedure. The authors argued that either the contrast medium or air embolism could have caused the cerebral injury. In a second report implicating vertebral artery injection, a patient developed respiratory and cardiovascular collapse shortly after a C6–C7 transforaminal injection of steroids and died in a coma 1 day later (69). A computed tomography (CT) scan revealed a large hemorrhage around the brainstem. A postmortem examination revealed cerebral edema, extensive hemorrhage in the brainstem and left cerebellum, and a thrombus in the left vertebral artery. A third patient developed quadriparesis after a right C5–C6 transforaminal injection and expired the
following day (70). No imaging or postmortem findings were provided.
following day (70). No imaging or postmortem findings were provided.
These case reports indicate that serious complications can occur as a result of unintentional intra-arterial injection in the course of cervical transforaminal injections. Either a radicular artery or the vertebral artery can be involved.
Published guidelines for the conduct of cervical transforaminal injections (63,71) are designed to guard against these complications (Table 50-4). The needle must be accurately and correctly placed against the posterior wall of the intervertebral foramen. In securing this position, it is essential that the needle be introduced along a correctly obtained oblique view of the target foramen (Fig. 50-2). Needle advancement using less than oblique or lateral views risks penetration of the vertebral artery en route to the foramen. Once the needle has been placed, a test dose of contrast medium should be injected and its flow carefully monitored during injection, using “live” or “real-time” fluoroscopy with or without digital subtraction. Under normal circumstances, the injectate should flow around the target nerve and into the lateral epidural space. Simultaneously, but more critically, this test dose of contrast shows if intravascular injection occurs. Close attention is required to notice if the injection is intra-arterial. The rapid flow through arteries means that intra-arterial contrast medium will appear only fleetingly. This event is unlikely to be captured by postinjection spot films. The flow of contrast medium must be monitored using continuous fluoroscopy throughout the injection.
Table 50-4 Preventing complications during cervical transforaminal injection | |
|---|---|
|
Lumbar Transforaminal Injections
Minor complications occurred in about 9% of a series of 322 lumbar transforaminal injections (72). Transient headaches (3%), increased back pain (2%), facial flushing (1%), increased leg pain (0.6%), and vasovagal reaction (0.3%) were the most frequently reported (Table 50-5). These complications are similar to those associated with lumbar interlaminar and caudal injections.
Similar to the major complications seen with cervical transforaminal injections, the major complications associated with lumbar transforaminal injections involve the reinforcing radicular artery, known as the artery of Adamkiewicz. Although this artery typically arises at thoracic levels, in 1% of individuals, this artery arises as low as L2, and more rarely as low as the
sacral levels (73). In those with a low-lying radicular artery, the artery can be entered during lumbar transforaminal injections (Fig. 50-4). There have been two reports of complications that likely resulted from direct injection into this vessel.
sacral levels (73). In those with a low-lying radicular artery, the artery can be entered during lumbar transforaminal injections (Fig. 50-4). There have been two reports of complications that likely resulted from direct injection into this vessel.
Table 50-5 Adverse effects associated with lumbar transforaminal injection | |
|---|---|
|
In one report, three patients developed paraplegia after lumbar transforaminal injections. In two cases the injections were performed at L3–L4 and in the third, the injection was at S1 (74). In all cases, MRI demonstrated increased signal intensity of the low thoracic spinal cord. A second report described one patient who developed paraplegia after an injection at L2–L3 (75). MRI showed increased signal intensity in the lower thoracic spinal cord and conus medullaris. The injection had been performed under CT guidance without a test dose of contrast medium.
The exact mechanism of spinal cord injury following lumbar transforaminal injections has not been determined. The pattern of injury seen on subsequent MRI strongly suggests occlusion of a reinforcing radicular artery. Both spasm of the artery or embolism of particulate steroids could account for these outcomes, but spasm seems less likely in light of the permanent and catastrophic outcomes. However, direct evidence is still lacking.
Published guidelines for lumbar transforaminal injections emphasize a technique similar to that used for cervical transforaminal injections (76). A test dose of contrast medium with real-time fluoroscopic monitoring is essential. Furthermore, during this test, a view of the lumbar spine that includes several segments cephalad of the level of injection should be used to assure that flow of contrast medium to the thoracic levels can be detected.
Neurologic complications of transforaminal injections are typically catastrophic. They are immediately obvious, with the onset of spinal weakness and numbness. Their recognition requires no special investigations. Magnetic resonance imaging of the spinal cord and hindbrain serves only to identify the location and extent of the neurologic damage. Immediate treatment is with ventilatory and cardiovascular support as needed, and subsequent management and rehabilitation follow standard protocols for spinal cord injury or stroke.
Complications Associated with Sympathetic Blocks
A number of acute, posttraumatic, and chronic neuropathic pain conditions are maintained through nociceptive impulses that travel through the sympathetic nervous system; these conditions often improve with sympathetic blockade. A recent evidence-based review (77) suggests that appropriate applications for sympathetic blocks include diagnosis of pain that is responsive to sympathetic blockade (e.g., complex regional pain syndrome or CRPS) and the treatment of ischemic pain. Pain arising from the viscera and sympathetically maintained pain (SMP) that arises after injury in the periphery can be effectively relieved in many instances by using local anesthetic or neurolytic blockade of the sympathetic nervous system at one of three main levels: the cervicothoracic ganglia (including the stellate ganglion); the celiac plexus; or the lumbar ganglia.
Sympathetic blocks including stellate ganglion, celiac plexus, and lumbar sympathetic blocks have been used for more than half a century. Practitioners have developed a relatively good understanding of the risks and complications of performing these procedures. Some newer techniques, such as hypogastric plexus block, and newer approaches to the sympathetic nerves, such as transdiscal approaches, have been described, but little is known about the risks of these approaches.
Stellate Ganglion Block
Complications from stellate ganglion block arise from vascular, epidural, and intrathecal injection (Table 50-6). Needle trauma can result in injuries to vessels or nervous tissue in the neck. Pneumothorax can also occur following a stellate ganglion block. Infections are uncommon. No published reports are available that offer an estimate of the frequency of complications associated with stellate ganglion block. In a recent
survey of members of the Canadian Anesthesiologists’ Society, approximately one-third of the anesthesiologists surveyed reported that they incorporate chronic pain treatment into their practice, and the most commonly practiced interventions included stellate ganglion block (61%) and lumbar sympathetic block (50%), suggesting that these techniques are commonly practiced. Most complications described, however, appear only in the form of sporadic case reports (see Chapter 39).
survey of members of the Canadian Anesthesiologists’ Society, approximately one-third of the anesthesiologists surveyed reported that they incorporate chronic pain treatment into their practice, and the most commonly practiced interventions included stellate ganglion block (61%) and lumbar sympathetic block (50%), suggesting that these techniques are commonly practiced. Most complications described, however, appear only in the form of sporadic case reports (see Chapter 39).
Table 50-6 Complications associated with stellate ganglion block | |
|---|---|
|
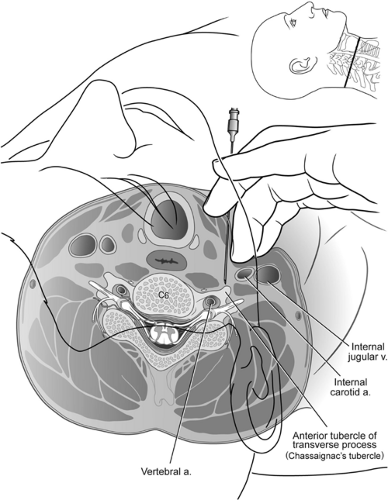
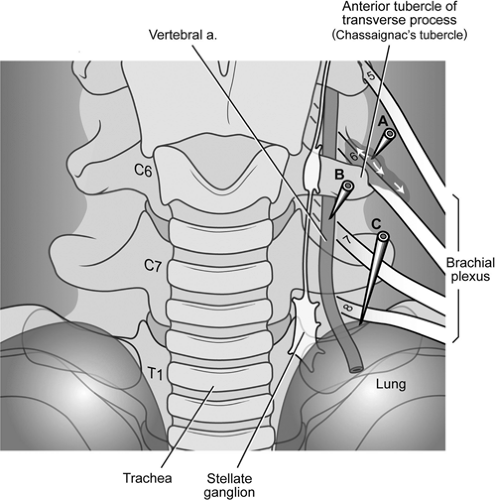
Several arteries and veins lie in close proximity to the intended injection site, the anterior tubercle of C6 or C7 (Fig. 50-5). Injections into the vein do not commonly result in sequelae since the volume and concentration of local anesthetic should not produce a toxic effect. The risk of local anesthetic toxicity can further be reduced by the use of dilute local anesthetic concentrations, such as 0.25% bupivacaine. Arterial injections do not offer the same measure of safety. As little as 2.5 mg of bupivacaine or a mixture of bupivacaine 1.25 mg and lidocaine 5 mg injected into the vertebral or internal carotid artery has been reported to produce seizures (78). Vertebral injections occur when the needle is inserted too medially and posteriorly. The practitioner often contacts bone but mistakes
the posterior tubercle for the anterior tubercle. Slight withdrawal of the needle from the posterior tubercle, particularly if in a medial position, can produce a vertebral artery injection. The carotid artery also lies near the site of entry for a stellate ganglion block. It is advisable to feel for the carotid pulse, then retract the vessel laterally prior to inserting the needle. This should prevent insertion into the carotid artery in most cases. Injection into the carotid artery can be expected to produce similar effects as vertebral artery injection.
the posterior tubercle for the anterior tubercle. Slight withdrawal of the needle from the posterior tubercle, particularly if in a medial position, can produce a vertebral artery injection. The carotid artery also lies near the site of entry for a stellate ganglion block. It is advisable to feel for the carotid pulse, then retract the vessel laterally prior to inserting the needle. This should prevent insertion into the carotid artery in most cases. Injection into the carotid artery can be expected to produce similar effects as vertebral artery injection.
Rarely is this procedure performed secondary to direct inflammation of the ganglion. Sympathetic blocks (e.g., stellate ganglion block) are performed diagnostically and therapeutically to denervate the sympathetic nerves. Local anesthetics effectively block these nerves temporarily. Long-term benefits have been reported following local anesthetic blocks. The addition of corticosteroids has not been demonstrated to enhance the effect of the local anesthetic. If there has been trauma at the site of the ganglion (e.g., a gunshot wound) then injection of corticosteroid with local anesthetic would seem reasonable. Routine administration of corticosteroid in these injections might be questioned because of the risk of intra-arterial injection into either the vertebral, carotid, or spinal radicular artery. Specifically, particulate corticosteroids (suspensions such as MPA) can cause an embolic stroke if unintentionally injected into any of these arteries.
Careful aspiration prior to injection helps to prevent intravascular injection but is not 100% effective. Slight movement of the needle can change the position from extravascular to intravascular. Attaching tubing to the needle and having a second person perform the aspirations and injections may further decrease the chance of intravascular injections, although no studies have been performed to confirm this. Incremental injections of local anesthetic injection are also advocated to minimize the chance of a large intravascular injection. If an intravascular (arterial) injection occurs, a grand mal seizure often results. Fortunately, these are transient and usually resolve prior to initiation of any therapy. Therapy is directed at maintaining an airway and preventing oral (teeth or tongue) trauma. Oxygen should be administered as soon as possible, although the seizure often ends before therapy can be initiated. The most serious risk from a grand mal seizure is aspiration of vomitus. For this reason, it is advisable to have a patient adhere to an “n.p.o.” status prior to the procedure. An IV line should be considered, particularly in patients with potentially difficult airways or larger than average necks (see also Chapter 39).
The cervical spinal nerves traverse the intervertebral foramen near the location for a stellate ganglion block (Fig. 50-6). If the needle is positioned posterior to the anterior tubercle, it can be positioned into either the epidural compartment or into a dural sleeve that accompanies the exiting nerve. Although CSF would be expected on aspiration if the needle were inside the subarachnoid space, this may be overlooked as the practitioner focuses on the more likely risk of vascular aspiration and injection. Paresthesias in the distribution of the brachial plexus may or may not occur. If present, one should consider repositioning the needle more anteriorly. Epidural injection of 10 mL of local anesthetic at the C6 or C7 level produces variable effects and is dependent on the concentration of local anesthetic and whether or not the entire volume of drug is injected. In our experience, epidural injection (with high concentrations of local anesthetic) can produce a profound sensory and motor block but often spares the phrenic nerve. Subjective respiratory distress is common secondary to block of the intercostal nerves. If airway reflexes are intact, continuous oxygen saturation monitoring and cardiac monitoring will allow the patient to be cared
for supportively (oxygen and IV fluids) and not require intubation. Small doses of benzodiazepines can be administered to allay the patient’s fears while the block subsides.
for supportively (oxygen and IV fluids) and not require intubation. Small doses of benzodiazepines can be administered to allay the patient’s fears while the block subsides.
Intrathecal injection of local anesthetic at this site commonly produces a total spinal block. Loss of airway reflexes and phrenic nerve function often occurs. Patients often describe difficulty breathing or an inability to move their arms as initial symptoms. Contralateral motor block develops as further confirmation, along with block of the lower extremities. Intubation and assisted ventilation will be required until the block abates. Blood pressure, cardiac, and oxygen-saturation monitoring should be performed until the block resolves. The duration of the block depends on the drug and the total dose injected. The patient will commonly remain awake during the event. Once the airway is protected and vital signs stabilized, some sedation to keep the patient comfortable is advised. Verbal reassurance of the patient can further calm fears and assure her that the effects she is experiencing are temporary.
Horner syndrome is often observed following a stellate ganglion block. In fact, many practitioners look for Horner syndrome as evidence of sympathetic denervation following the stellate ganglion injection of local anesthetic. Horner syndrome is, theoretically, a side effect, since a stellate ganglion block is most commonly performed for patients with upper extremity pain (diagnostically to rule in SMP or therapeutically for CRPS type I or II). Horner syndrome consists of miosis (papillary constriction), ptosis (drooping of the upper eyelid), and enophthalmos (recession of the globe within the orbit). The presence of Horner syndrome does not necessarily equate to sympathetic denervation of the upper extremity. A study by Malmqvist et al. used five measures of sympathetic denervation (including the presence of Horner syndrome, increased temperature of the blocked extremity, and sympathogalvanic skin response) to evaluate the effectiveness of a stellate ganglion block (79). The authors found only 15 of 54 blocks had four of five positive measures following stellate ganglion block. A study by Hogan et al. examined the distribution and spread of contrast following stellate ganglion block using MRI (80). They found that injectate was not delivered to the stellate ganglion but rather passed anterior to it (with or without caudad extension to the stellate ganglion). This could produce Horner syndrome but not sympathetic denervation to the extremity. Other sites of spread included the brachial plexus, the subclavian plexus, and the epidural or subarachnoid spaces. Horner syndrome following stellate ganglion injection with local anesthetic resolves when the local anesthetic effect ends. Ideally, the analgesic effect outlives the Horner syndrome. Use of neurolytic solutions can produce permanent Horner syndrome when injected near the stellate ganglion. Use of dilute neurolytic solutions has been reported by Racz et al. as safe, in that they may not produce permanent Horner syndrome (personal communication). However, the use of neurolytic solutions near the stellate ganglion cannot be advocated for routine cases and should be used only in special situations following clear discussions of risks and benefits with the patient.
Recurrent laryngeal and phrenic nerve blocks are frequent side effects of a stellate ganglion block. They occur from local anesthetic injection that spills from the area of the ganglion. Since diffusion of drug is required to obtain a satisfactory block, it can be expected that these nerves will often be temporarily blocked. Symptoms of a recurrent laryngeal nerve block include hoarseness and, occasionally, respiratory stridor. Patients often complain of difficulty in getting their breath and the sensation of a lump in their throats. Symptomatic treatment is sufficient along with reassurance that these sensations will resolve as the local anesthetic dissipates. Patients should be cautioned to drink clear liquids initially after a stellate ganglion block to make sure that their upper airway function is not compromised. Once they feel comfortable swallowing liquids, they can progress to regular food. Phrenic nerve block rarely presents a problem for patients unless they have preexisting severe respiratory compromise. The potential for producing phrenic nerve block is one reason that bilateral stellate ganglion blocks are rarely performed at the same time. Most practitioners wait several days or a week between injections.
The pleural dome of the lung extends variably above the first rib. Most commonly, this dome passes laterally to the intended C6 injection site for the stellate ganglion block (Fig. 50-6). Using the anterior C7 approach to the stellate ganglion, the pleural dome is closer and may be entered in unusual cases. This can be accentuated in patients with pulmonary disease and hyperinflated lungs, such as patients with chronic obstructive pulmonary disease (COPD). The posterior approach to the upper thoracic sympathetic chain is extremely close to the parietal pleura. Anyone using this approach should be cautious and guided by fluoroscopy or CT techniques. Pneumothorax can result despite careful attention to detail, and patients should be warned of this potential complication prior to a posterior approach of the sympathetic chain or stellate ganglion.
If air is aspirated during placement of the needle, the clinician should decide if the trachea or pulmonary parenchyma has been entered. Breath sounds should be examined following the procedure if one suspects a pulmonary problem. Any abnormalities should be followed up with a chest radiograph. Inspiration and expiration films should be considered. Delayed presentation of a pneumothorax is always possible. If a pneumothorax is suspected but unconfirmed, patients should be warned to call or go to an emergency facility if they become symptomatic. Patients who develop a pneumothorax should be hospitalized and watched closely, with appropriate consultation with a respiratory physician or a cardiothoracic surgeon, if indicated.
Brachial plexus block can occur following a stellate ganglion injection. The majority of sympathetic nerves that travel to the upper extremity leave the sympathetic chain to accompany the somatic nerves of the brachial plexus. A few sympathetic nerves of the upper extremity, often referred to as the anomalous Kuntz nerves, may not pass through the stellate ganglion but later join the brachial plexus (81). These upper extremity sympathetic fibers can only be blocked by either a brachial plexus block or a posterior approach to the upper thoracic sympathetic chain. In a standard, anterior (C6) approach to the stellate ganglion, the brachial plexus will not be blocked. However, partial block of the nerve roots that form the brachial plexus is commonly seen after a stellate ganglion block. This occurs most commonly when the needle has been inserted too deeply, bypasses the anterior tubercle, and rests on the posterior tubercle. After retraction from the posterior tubercle, the injection of local anesthetic is likely to block one or more of the exiting nerve roots of the brachial plexus.
There are no long-term sequelae of a block of the brachial plexus with local anesthetic. An intraneural injection is almost impossible, but extreme radiating pain during injection should be avoided in the unlikely event that the needle has pierced the epineurial layer of a nerve. Outpatients who develop a partial brachial plexus motor block should be warned about being insensate for possibly 24 hours or more (dependent on the volume and concentration of local anesthetic used) and should avoid putting the extremity in an unsafe position, such as one in which a burn could occur. Use of a sling may help diminish any risk associated with a motor block.
Partial brachial plexus block may make interpretation of the stellate ganglion block difficult. Complete sympathetic denervation may not coexist if the stellate ganglion is not equally blocked or if the entire brachial plexus denervated. Patients can also be distracted by the motor block and experience dysesthesias, anesthesia dolorosa, and the like. Although complete brachial plexus denervation can be an excellent therapeutic modality for patients with severe dystonia and CRPS, partial block following stellate ganglion block should be viewed as an undesired, although most frequently harmless, side effect.
Paratracheal hematoma causing airway obstruction and death has been reported after stellate ganglion block (82). This may occur if the vertebral artery or carotid artery has been entered. In the event of a hematoma, direct pressure should be held. Communication with the patient should continue to make sure that there is no compromise of arterial blood flow to the cerebral cortex while pressure is maintained. A more serious, although very rare, situation develops if a plaque is dislodged following unintentional arterial puncture of either the carotid or vertebral artery. Presentation would be expected to mimic an evolving stroke. Similarly, unintentional puncture can also produce an arterial wall dissection. This is more likely in the rare situation in which the needle rests in the wall of the artery, negative aspiration is noted, and subsequent injection of the local anesthetic produces the dissections. This should be viewed as a medical emergency, with life care support and direct transport to an emergency facility indicated.
Infections are very uncommon following stellate ganglion injections, but case reports of cervical vertebral osteomyelitis do exist (83,84). Risk of infection is minimized by use of alcohol preparation of the site and good technique with sterile surgical gloves. More formal surgical preparation using Betadine or a similar solution and sterile drapes can also be recommended. Many clinicians believe it is important, however, to watch the patient during the injection for immediate feedback if an intravascular injection occurs and therefore recommend avoiding the use of drapes over the patient’s face. If an infection occurs, the most likely organism would be a strain of Staphylococcus unless the esophagus was inadvertently entered. Since the needle can contact bone during the procedure, any infection should be treated with antibiotics and followed closely. An infectious disease consult should be considered in patients who do not respond to a course of oral antibiotics.
Two separate case reports have reported a transient locked-in syndrome following stellate ganglion block (85,86). In both cases, the patients remained conscious but were unable to breathe or move, with sparing of eye movement. The authors of both reports believed these cases were the result of intra-arterial injections of the vertebral artery. In our practice, we have seen two cases of a similar presentation in 20 years. However, we attributed both cases to a total spinal injection. Whether these cases represent a different phenomenon or a misdiagnosis is unclear. Fortunately, in all four cases (ours and the published cases), the patients recovered uneventfully. Care should be taken to provide sedation and frequent reassurance to these patients, as they are conscious but unable to move or breathe.
Wallace and Milholland have reported a case of contralateral and bilateral Horner syndrome following stellate ganglion injection (87). They also reported a bilateral recurrent laryngeal nerve block, which can potentially produce life-threatening airway compromise and must be monitored closely.
Intubation may be required if patients cannot sustain acceptable oxygen saturation.
Kimura et al. have reported severe hypertension following stellate ganglion block (88). They reported seven patients who developed a systolic blood pressures of greater than 200 mm Hg after a stellate ganglion block. They postulated that local anesthetic diffused along the carotid sheath, resulting in block of the vagus nerve, attenuation of the baroreceptor reflex, and subsequent unopposed sympathetic activity.
Lumbar Sympathetic Block
Lumbar sympathetic block is commonly performed for either diagnostic or therapeutic purposes in patients with suspected SMP in the lower extremities and for vascular ischemia (see Chapter 39). No published data present any estimate of the frequency of complications associated with this block, and the complications described here are derived from case reports. Complications that have been reported with lumbar sympathetic block include intravascular injection, intraspinal injection, infection (including discitis), postdural puncture headache (PDPH), and hematoma formation (Table 50-7).
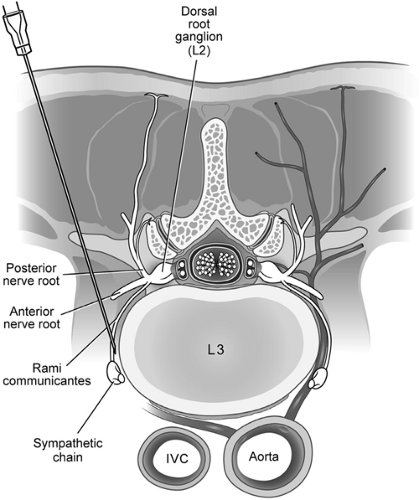
On the left side of the spine, the aorta lies close to the needle placement site for a lumbar sympathetic block, and the inferior vena cava is near the placement site for needles placed on the right side (Fig. 50-7). Large volumes of local anesthetic, particularly bupivacaine, injected into either vessel can cause seizures and/or cardiovascular collapse if injected into either vessel. Resuscitation can be prolonged and difficult if bupivacaine is injected. Careful, frequent aspiration and intermittent injection is recommended. If the solution contains epinephrine, changes in heart rate may serve as a signal of intravascular injection.
It is possible for the needle to come to rest in a nerve root sleeve, with final placement in either the epidural or intrathecal space, although this occurs less frequently now that fluoroscopy is commonly used during these procedures. Entry into the spinal canal when using the “blind” technique (i.e., using surface landmarks alone to guide needle placement) occurs when the angle of needle placement is too shallow and aims directly toward the intervertebral foramen rather than the anterolateral surface of the vertebral body. Intraspinal injection should occur rarely, if ever, with the proper use of radiographic guidance during LSB. If a local anesthetic is the only drug used, side effects should resolve over time. Supportive care and vasopressors may be required.
Neurolytic lumbar sympathetic block has been advocated in efforts to produce long-lasting pain relief in those patients who receive temporary relief following local anesthetic injection. Fluoroscopic use should decrease the incidence of complications from LSB injections. A comparison of alcohol and phenol demonstrated that alcohol blocks were more likely to produce an L2 neuralgia than was phenol. However, either agent can produce an L2 neuralgia, and this complication can occur with good spread of contrast (see Chapter 39). For this reason, some
practitioners advocate the use of radiofrequency denervation. Multiple lesions are required using radiofrequency to achieve adequate denervation (89). There is insufficient published information on the radiofrequency technique to understand if it will reduce the incidence of neuralgia; however, neuralgia has been reported in several cases following neurolytic LSB using radiofrequency (89).
practitioners advocate the use of radiofrequency denervation. Multiple lesions are required using radiofrequency to achieve adequate denervation (89). There is insufficient published information on the radiofrequency technique to understand if it will reduce the incidence of neuralgia; however, neuralgia has been reported in several cases following neurolytic LSB using radiofrequency (89).
Table 50-7 Complications of Lumbar Sympathetic Block | |
|---|---|
|
The intervertebral disc lies near the path of a LSB needle (Fig. 50-7). It is not uncommon for the needle to unintentionally pass through part of the disc. Normally, this does not result in a complication, but discitis can occur. Historically, complete surgical drape, gown, and other precautions are not used for a LSB (as one might for a discogram). Whether this increases the risk of discitis is unclear. In modern practice, sterile technique should be used for LSB. Also, it would seem prudent to avoid the intervertebral disc whenever possible, given the potentially serious consequences of discitis. Our practice has seen one serious discitis from a “blind” LSB that was done before fluoroscopy was in common use. The patient ultimately required surgery and partial vertebrectomy to resolve the infection.
Severe bleeding following LSB was reported in two patients: a large subcutaneous hematoma in one case, and a massive retroperitoneal hematoma in the second (90). Both patients were receiving irreversible platelet aggregation inhibitors (ticlopidine or clopidogrel). The considerations for performing sympathetic blocks in patients receiving these agents are similar to those proposed for neuraxial blockade (44). Discontinuing use of anticoagulants or other drugs used to inhibit platelet aggregation carries its own set of risks. Patients have suffered embolic strokes when appropriately stopped from their anticoagulant medications prior to interventional procedures. The risk–benefit ratio of stopping or continuing any of these drugs should be carefully considered. Alternative therapies may be appropriate. Consulting the patient’s primary care physician or the specialist who is prescribing the anticoagulant is indicated.
Post–dural puncture headache after LSB has been reported in two cases (91). The cases most likely occurred as a result of the needle passing near a nerve root sleeve that contained spinal fluid. One patient underwent an unsuccessful attempt at an epidural blood patch. Needles that are placed too laterally and posteriorly will come to rest in the psoas sheath or muscle. A striated appearance on fluoroscopy is indicative of needle placement into the muscle. If local anesthetic is injected, patients will develop a motor block of the femoral plexus, with resultant lower extremity weakness. Renal trauma or puncture of a ureter can occur with needles that begin too far laterally. Most practitioners avoid inserting the needle more than 7 to 8 cm from the midline. Fortunately, sequelae are minimal unless a neurolytic agent is injected, resulting in possible ureteral stricture or extravasation of urine.
Celiac Plexus Block
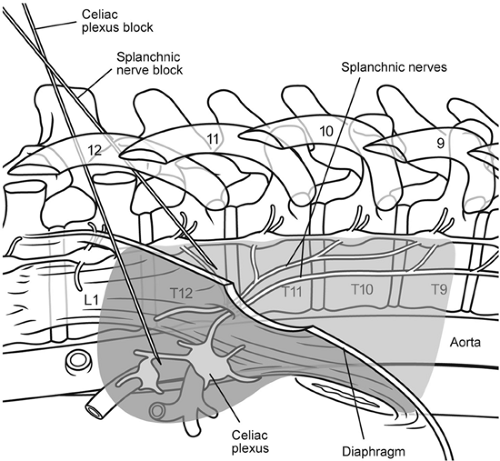
Neurolytic celiac plexus block (NCPB) has been shown to be an effective analgesic technique for management of pain in patients with intra-abdominal malignancies, especially pancreatic cancer (92,93,94,95). The technique of NCPB involves injection of neurolytic solutions in the area of the celiac plexus or splanchnic nerves, which are neural structures that transmit the majority of visceral pain from the upper abdomen (96). The celiac plexus is comprised of a diffuse network of nerve fibers and individual ganglia that lie over the anterolateral surface of the aorta, primarily at the T12–L1 vertebral level. Sympathetic innervation to the abdominal viscera arises from the anterolateral horn of the spinal cord between the T5 and T12 levels. Nociceptive information from the abdominal viscera is carried by afferents that accompany the sympathetic nerves. Presynaptic sympathetic fibers travel from the thoracic sympathetic chain toward the ganglia, traversing over the anterolateral aspect of the inferior thoracic vertebrae as the greater (T5–T9), lesser (T10–T11), and least (T12) splanchnic nerves (Fig. 50-8). Presynaptic fibers traveling via the splanchnic nerves synapse within the celiac ganglia, over the anterolateral surface of the aorta surrounding the origin of the celiac and superior mesenteric arteries at approximately the L1 vertebral level. Post-synaptic fibers from the celiac ganglia innervate the upper abdominal viscera, with the exception of the descending colon, sigmoid colon, rectum, and pelvic viscera.
Celiac plexus block using a transcrural approach places the local anesthetic or neurolytic solution in direct contact with the celiac ganglion anterolateral to the aorta (Fig. 50-8). The needles pass directly through the crura of the diaphragm en route to the celiac plexus. Because of the diaphragm, spread of the solution toward the posterior surface of the aorta may be more limited, perhaps reducing the chance of nerve root or spinal segmental artery involvement. In contrast, splanchnic nerve block (Fig. 50-8) avoids the risk of penetrating the aorta, uses smaller volumes of solution, and is unlikely to be affected by anatomic distortion caused by extensive tumor of the pancreas or metastatic lymphadenopathy. Because the needles remain posterior to the diaphragmatic crura in close apposition to the T12 vertebral body, this has been termed the retrocrural technique. Splanchnic nerve block is a minor modification
of the classic retrocrural celiac plexus block, the only difference being that, for splanchnic block, the needles are placed over the midportion of the T12 vertebral body rather than the cephalad portion of L1. Retrocrural celiac plexus block at the superior aspect of the L1 vertebral body and splanchnic nerve block at the mid T12 vertebral body have both been described, and they are essentially the same technique, both relying on cephalad spread of solution to block the splanchnic nerves in a retrocrural location. In most cases, celiac plexus (transcrural or retrocrural) and splanchnic nerve block can be used interchangeably to produce the same results. Although there are those who strongly advocate one approach or the other, there is no evidence that either results in superior clinical outcomes (95). From the perspective of complications in this chapter, NCPB may refer to neurolytic blockade of either celiac plexus or splanchnic nerves (see also Chapter 45).
of the classic retrocrural celiac plexus block, the only difference being that, for splanchnic block, the needles are placed over the midportion of the T12 vertebral body rather than the cephalad portion of L1. Retrocrural celiac plexus block at the superior aspect of the L1 vertebral body and splanchnic nerve block at the mid T12 vertebral body have both been described, and they are essentially the same technique, both relying on cephalad spread of solution to block the splanchnic nerves in a retrocrural location. In most cases, celiac plexus (transcrural or retrocrural) and splanchnic nerve block can be used interchangeably to produce the same results. Although there are those who strongly advocate one approach or the other, there is no evidence that either results in superior clinical outcomes (95). From the perspective of complications in this chapter, NCPB may refer to neurolytic blockade of either celiac plexus or splanchnic nerves (see also Chapter 45).
Overall, the NCPB is considered to be a relatively safe procedure in clinical practice. Despite the infrequency of significant complications, there is still a range of potential complications associated with NCPB that must be considered, including cardiovascular, gastrointestinal, genitourinary, pulmonary, systemic, and neurologic (Table 50-8).
Many of the minor complications of NCPB are the result of effective sympathetic block due to neurolysis of the celiac ganglia. These side effects can usually be treated in a symptomatic manner without a significant effect on the patient. For example, orthostatic hypotension typically improves shortly after equilibration of the intravascular volume. Another example, bowel hypermotility, is usually transient, and may actually be desirable in many patients with opioid-induced constipation.
Major complications are often related to significant neurologic deficits or vascular events, but are extremely uncommon. Neurologic complications, such as loss of sensation or motor function of the lower extremities, are very uncommon, but can have significant clinical impact and lasting duration (97). In patients with intra-abdominal malignancies, these resultant neurologic deficits often continue for the remainder of their lives. Serious vascular events may involve uncontrolled arterial bleeding or aortic dissection (98,99), which may be life- threatening.
Most cases of complications and derived frequencies of complications are based on patients receiving NCPB from a posterior approach. The celiac plexus and splanchnic nerves are primarily sympathetic nervous system structures. Neurolytic blockade of these sympathetic structures results in a relative increase in parasympathetic tone to the splanchnic region. Therefore, vasodilation of the splanchnic vasculature can result in orthostatic hypotension, and relative increase in parasympathetic tone may cause bowel hypermobility (see Chapter 45).
Meta-analysis of the literature regarding patients undergoing NCPB found that hypotension occurred in 38% of cases and bowel hypermotility occurred in 44% of cases (100). A case series of 136 patients who had NCPB reported that eight patients (6%) with symptomatic orthostatic hypotension required treatment (101). Another study (61 patients), prospectively comparing different CPB techniques, reported a 38% incidence of transient decreases in systolic blood pressure of more than 33% compared to baseline measurements (95). Bowel hypermotility also occurred in 31% of these patients. In the largest series (2,730 patients) evaluating neurologic complications associated with NCPB, four cases (0.15%) of permanent paraplegia were identified (97). In three of these cases, loss of anal and bladder sphincter function also occurred. Radiographic guidance with radiocontrast dye was used for CPB in all four cases. In a case series by Brown et al., there were no cases of permanent paraplegia in 136 patients with NCPB (101). Meta-analysis of the literature revealed a 1% incidence of neurologic complications, including lower extremity weakness, paresthesia, epidural anesthesia, and lumbar puncture, after NCPB (100).
Complications of celiac plexus and splanchnic nerve block include hematuria, intravascular injection, and pneumothorax.
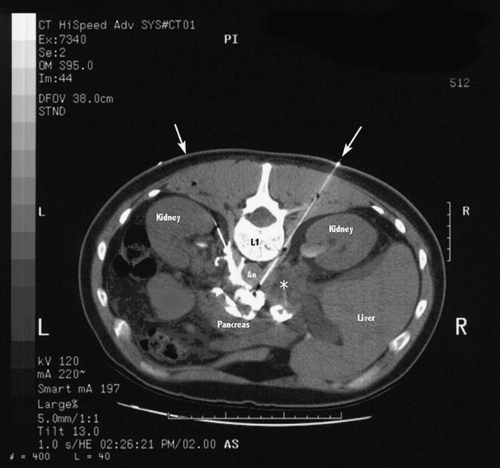
Computed tomography (CT) allows visualization of the structures located adjacent to the celiac ganglia as the block is being performed (Fig. 50-9). The kidneys extend from between T12 and L3, with the left kidney slightly more cephalad than the right. The aorta lies over the left anterolateral border of the vertebral column. Cardiovascular-related complications may include needle puncture injury resulting in aortic or major arterial bleeding (98,99). The inferior vena cava lies just to the right of the aorta, over the anterolateral surface of the vertebral column. The medial pleural reflection extends inferomedially as low as the T12–L1 level.
Computed tomography (CT) allows visualization of the structures located adjacent to the celiac ganglia as the block is being performed (Fig. 50-9). The kidneys extend from between T12 and L3, with the left kidney slightly more cephalad than the right. The aorta lies over the left anterolateral border of the vertebral column. Cardiovascular-related complications may include needle puncture injury resulting in aortic or major arterial bleeding (98,99). The inferior vena cava lies just to the right of the aorta, over the anterolateral surface of the vertebral column. The medial pleural reflection extends inferomedially as low as the T12–L1 level.
Table 50-8 Summary of complications associated with celiac plexus block, their clinical signs and symptoms, diagnostic evaluation, treatment, and management | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Gastrointestinal-related complications such as bowel hypermotility (100) can occur frequently from effective sympathetic block due to neurolysis of the celiac plexus. The blockade of sympathetic outflow to the viscera during continued parasympathetic outflow to the viscera is likely to result in increased peristalsis and bowel hypermotility. Another complication, the loss of anal sphincter control, could result from the inadvertent neurolysis of relevant central and/or peripheral nerves (97). The mechanism of injury in this report is unclear; one can only speculate that neurolytic solution might have tracked either centrally (epidural or intrathecal) to a minor extent or contacted one of the sacral roots. Genitourinary-related complications can occur due to needle puncture of the kidney (102). Separately, contact of neurolytic solution with relevant central and/or peripheral nerves can result in loss of bladder control (97) and impotence in males (103). Pulmonary-related complications are infrequent but are related to pneumothorax resulting from needle puncture traversing from the back passing excessively anteriorly (101).
Acetaldehyde dehydrogenase deficiency results in sensitivity to ethanol, which is frequently used for neurolysis (104). Following ethanol injection, individuals with aldehyde dehydrogenase deficiency have increased systemic levels of acetaldehyde, causing skin flushing, nausea, headache, tachycardia, hypotension, and drowsiness (105) (see also Chapter 45).
Neurologic complications involve sensory and/or motor deficits of the lower extremities. Although the incidence is extremely infrequent, these neurologic deficits often represent the most worrisome complications associated with NCPB. Different mechanisms are possible, with the most likely cause involving the injection or contact of neurolytic solutions with neural structures other than the celiac ganglia or splanchnic nerves. Possible routes could include inadvertent needle placement or injection into the intrathecal or epidural space, thoracic or lumbar nerve roots, or lumbar plexus within the psoas muscle compartment. Another separate mechanism involves the interruption of blood flow due to injury or spasm of the major anterior radicular artery, also known as the artery of Adamkiewicz, which provides blood flow to the anterior spinal artery (106,107) (Fig. 50-10). Interruption of blood flow to the spinal cord can result in permanent sensory and motor deficits of the lower trunk and/or extremities.
Some of the complications associated with NCPB may be difficult to prevent even in the care of an experienced pain medicine specialist. These complications include needle puncture injuries, complications related to sympatholysis from an effective block (e.g., bowel hypermotility), unexpected drug sensitivities, and vascular spasm of the major anterior radicular artery (artery of Adamkiewicz).
Radiographic guidance, such as fluoroscopy or CT, is commonly used in contemporary practice to recognize grossly inaccurate needle placement. The use of radiographic guidance may provide improved visualization of anatomic structures relevant to the NCPB. Improved sense of the anatomy may lower the risk of some complications due to incorrect needle direction or placement. Radiocontrast dye can confirm appropriate needle position based on appropriate spread of injected solution. Despite its apparent usefulness, it is important to consider that radiographic guidance cannot provide complete prevention of neurologic complications. In the large case series of 2,730 patients with NCPB, four cases of permanent paraplegia occurred. All four cases of paraplegia involved the use of radiography and radiocontrast dye to confirm final needle placement. Nonetheless, there seems to be consensus in the specialty for the use of radiographic guidance for NCPB in the contemporary practice of interventional pain medicine.
Minor complications, such as orthostatic hypotension and bowel hypermotility, are common and typically self-limited. Orthostatic hypotension, if symptomatic, can be treated by increasing oral fluid intake or by IV fluids. Leg wrappings with elastic bandages or support stockings can also decrease venous capacitance and improve hypotension. Short-term use of oral ephedrine tablets 15 to 30 mg/q8h may be useful at times to allow patients to ambulate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree