2 Commonly Used Medications in Procedures
Local Anesthetics
Mechanism of Action
In addition to host factors, neural blockade by local anesthetics is affected by the volume and concentration of local anesthetic injected, the absence or presence of vasoconstrictor additives, the site of injection, the addition of bicarbonate, and temperature of the local anesthetic.1 Increasing the total milligrams of a local anesthetic dose shortens the onset and increases the duration of the local anesthetic. Epinephrine, norepinephrine, and phenylephrine are sometimes added to local anesthetics to reverse the intrinsic vasodilation effects of many of the local anesthetics and thereby reduce their systemic absorption. This increases the amount of local anesthetic available to block the nerve. More anesthetic means a quicker onset and longer duration. Application of the local anesthetic close to the nerve improves its ability to diffuse across the axon and block sodium channels. Highly vascular sites such as the intercostal nerve and caudal epidural space tend to result in slightly shorter duration of action. The addition of bicarbonate or CO2 (700 mm Hg) to local anesthetics hasten their onset. Bicarbonate raises the pH and the amount of uncharged local anesthetic for diffusion through the nerve membrane. CO2 will diffuse across the axonal membrane and lower the intracellular pH making more of the charged form of the local anesthetic available intracellularly to block the sodium channels. Temperature elevations decrease the pKa of the local anesthetic and hasten the onset of action.
Individual Agents
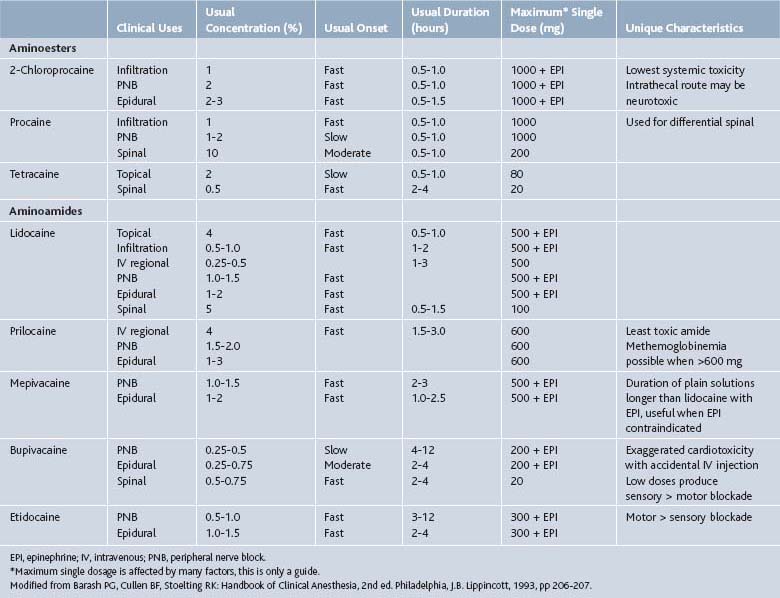
Local anesthetics are administered in the intradermal, subcutaneous, intraarticular, intramuscular, perineural, and epidural spaces during pain management procedures. Injections into vascular regions such as the oral mucosa and epidural space may result in rapid absorption and higher systemic concentrations. Local anesthetics administered into or near the epidural space should be preservative free. Methylparaben is a common preservative in multidose vials and is also a common allergen.2
Lidocaine
Lidocaine is the most versatile and widely used of the local anesthetics. It has a short onset of action, 0.5 to 15 minutes, and short duration of action, typically 0.5 to 3 hours. The difference between the effective dose and the toxic does is wide, resulting in a high therapeutic index compared to other common local anesthetics. Maximum doses are variably reported in the range of 400 to 500 mg of lidocaine. Typical concentrations are 0.5% to 2%. Final concentration is often diluted by the addition of a corticosteroid.1
Bupivicaine
Bupivacaine (Marcaine) is another widely used local anesthetic. Bupivacaine’s duration of action (2 to 5 hr) is longer than lidocaine’s as is its onset of action (5 to 20 min). Bupivacaine is commonly used in concentrations of 0.125% to 0.75%. Final concentrations are often diluted by 30% to 50% by the addition of a corticosteroid. The higher concentrations generally have a faster onset of action. Bupivacaine has more cardiotoxicity than lidocaine, especially if an injection is given intravenously inadvertently. The toxic dose of bupivacaine is only 80 mg (16 mL of a 0.5% solution) when given intravascularly, but may be up to 225 mg with an extravascular injection.1
Toxicity
Action of local anesthetics is affected by numerous factors reviewed above. Location of injection plays a primary role in determining the onset, duration, and toxic dose of these agents (Table 2-1). Vasoconstrictors such as epinephrine reduce local bleeding and thereby prolong the onset and duration, but are generally not employed in a pain management practice.
Patients should be monitored for signs of toxicity including restlessness, anxiety, incoherent speech, lightheadedness, numbness, and tingling of the mouth and lips, blurred vision, tremors, twitching, depression or drowsiness. Injections into the head and neck area require the utmost care.3 Even small doses of local anesthetic may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Deaths have been reported.4
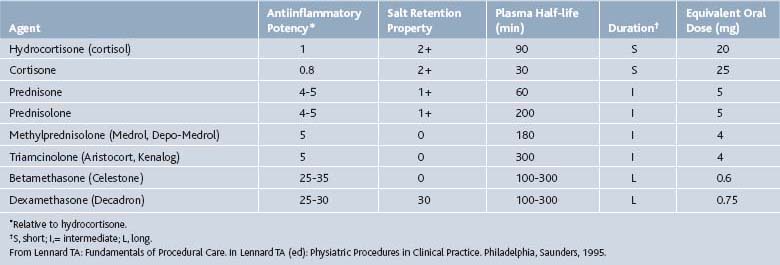
Corticosteroids
Corticosteroids can be helpful in a variety of conditions including rheumatoid arthritis, bursitis, tenosynovitis, entrapment neuropathies, crystal-induced arthropathies in patients who cannot tolerate systemic treatment well, radiculopathies, and at times, osteoarthritis (OA). Corticosteroids should never be injected directly into a tendon or nerve, subcutaneous fat, or an infected joint, bursa, or tendon (Table 2-2).
Mechanism of Action
Corticosteroids produce both antiinflammatory and immunosuppressive effects in humans. The primary mechanism of action may be their ability to inhibit the release of cytokines by immune cells.5 The effects of corticosteroids are species specific.6 Lymphocytes in humans are much less sensitive to the effects of corticosteroids than lymphocytes in common laboratory animals including the mouse, rat, and rabbit. In humans, corticosteroids reduce the accumulation of lymphocytes at inflammatory sites by a migratory effect.7 In contrast to this lymphopenia, is the neutrophilia seen by demargination of neutrocytes from the endothelium and an accelerated rate of release from the bone marrow.8 A temporary rise in white blood cell count is commonly observed for this reason after a corticosteroid dose and in isolation does not mark a post injection infection.
The antiinflammatory effects of corticosteroid also occur at the microvascular level. They block the passage of immune complexes across the basement membrane, suppress superoxide radicals, and reduce capillary permeability and blood flow.9 Corticosteroids inhibit prostaglandin synthesis,10 decrease collagenase formation, and inhibit granulation tissue formation.
The immunosuppressant effects of corticosteroids are generally via effects on T cells. These effects are not the desired effect of corticosteroid used in pain management procedures and are not observed following epidural injections.11 A review of these immunosuppressant effects can be found in other texts.11–14
Individual Agents
Betamethasone
An equal mixture of two betamethasone salts, Celestone Soluspan, allows for both immediate and delayed corticosteroid responses. Betamethasone sodium phosphate acts within hours, whereas betamethasone acetate is a suspension that is slowly absorbed over approximately 2 weeks. Betamethasone (Celestone Soluspan) is approved for intraarticular or soft tissue injection to provide short-term adjuvant therapy in osteoarthritis, tenosynovitis, gouty arthritis, bursitis, epicondylitis, and rheumatoid arthritis.15 It is also commonly employed in epidural injections. Typical intraarticular doses vary with the size of the joint and range from 0.25 to 2 mL (1.5 mg to 12 mg). Typically epidural injections range from 1 to 3 mL (6 to 18 mg). Betamethasone should not be mixed with local anesthetics that contain preservatives such as methylparaben as these may cause flocculation of the steroid.
Dexamethasone
Dexamethasone sodium phosphate (Decadron Phosphate) is a rapid onset, short duration formulation of dexamethasone. It is available in a variety of strengths ranging from 4 mg/mL to 24 mg/mL. Large joints are often injected with 2 to 4 mg, small joints 0.8 to 1 mg, bursae 2 to 3mg, tendon sheaths 0.4 to 1mg, soft tissue infiltration 2 to 6 mg.15 Sulfites are common in the preparations of this salt also. Dexamethasone is approved for the treatment of osteoarthritis, bursitis, tendonitis, rheumatoid arthritis flares, epicondylitis, tenosynovitis, and gouty arthritis.15 Because it is considered to be a nonparticulate steroid it is also used off-label for epidural steroid injections as discussed subsequently.
Methylprednisolone
Methylprednisolone acetate (Depo-Medrol) has 1/5 to 1/6 the glucocorticoid potency of betamethasone but similar antiinflammatory effects to prednisolone. It has an intermediate duration of action. It, like the other corticosteroids, is approved for intraarticular and soft tissue injections for short-term adjuvant therapy of osteoarthritis, bursitis, tenosynovitis, gouty arthritis, epicondylitis, and rheumatoid arthritis.15 Depo-Medrol has been used for epidural administration also. Preparations of methylprednisolone acetate include polyethylene glycol as a suspending agent. Concerns developed as to whether the polyethylene glycol can cause arachnoiditis with (inadvertent) intrathecal injections.16 Animal studies have not demonstrated any adverse effects on neural tissues from the application of glucocorticoid.17 Methylprednisolone is now available without polyethylene glycol, PEG free. Typical doses range from 4 to 80 mg. Small joints are typically injected with 4 to 10 mg, medium joints 10 to 40mg, large joints 20 to 80 mg, bursae and peritendon 4 to 30 mg.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree