Common Adult Congenital Heart Diseases
Carlos A. Roldan
Common adult congenital heart diseases (CHD) include atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), and coarctation of the aorta (1).
The accurate diagnosis and assessment of the hemodynamic significance of CHD are necessary in determining their need and type of therapy and preventing their associated morbidity and mortality.
The history and physical examination are valuable in the detection of CHD but are limited in defining their severity and type of therapy for a defect.
Indications for Echocardiography
Two-dimensional (2D) and currently real-time three-dimensional (RT3D) transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE), and intracardiac echocardiography (ICE), are essential in the diagnosis, characterization, severity assessment, and management of CHD (2,3,4,5) (Table 12.1).
Atrial Septal Defect
Definition and Classification
A discontinuity of the interatrial septum classified into secundum ASD, primum ASD, sinoseptal ASD, and patent foramen ovale (PFO).
Secundum ASD, the most common defect (70% of all defects), is caused by a deficient septum primum that fails to cover the ostium secundum, located centrally in the fossa ovalis area, and usually single.
Primum ASD, which accounts for approximately 15% to 20% of defects, is caused by failure of the endocardial cushions to meet the atrial septum, always associated with a cleft anterior mitral leaflet, and located posteroinferior to the aortic root.
Sinoseptal defects include the sinus venosus defects and the unroofed coronary sinus:
The superior vena cava (SVC) sinus venosus ASD, the most common sinoseptal defect (5% to 10% of all defects), occurs at the most posterosuperior portion of the atrial septum at the junction of the SVC with the right atrium (RA), and it is almost always associated with anomalous drainage of the right upper and middle pulmonary veins into the SVC or RA.
Inferior vena cava (IVC) sinus venosus defects are uncommon, are located posteroinferior to the fossa ovalis at the IVC/RA junction, and are associated with anomalous drainage of the right lower pulmonary vein.
An unroofed coronary sinus is a rare defect of the coronary sinus in the left atrium (LA), allows flow from the LA to the RA, and is often associated with a persistent left SVC (Raghib anomaly).
A PFO is present in 8% to 20% of the general population, and in contrast to an ASD, a PFO is generally <5 mm in diameter and allows minimal unidirectional shunting from the RA to LA.
Prevalence
Table 12.1 Class I or appropriate (score 7–9) indications for echocardiography in the adult with suspected or known congenital heart disease | ||
|---|---|---|
|
Echocardiography
Morphology of Atrial Septal Defects on M-Mode, Two-dimensional and Real-time Three-dimensional Transthoracic and Transesophageal Echocardiography, and Intracardiac Echocardiography
Best Imaging Planes
Two-dimensional TTE parasternal short-axis view at the aortic level and apical and subcostal four-chamber views to identify secundum and primum ASD.
Two-dimensional TTE right parasternal long- and short-axis and subcostal views to identify sinus venosus ASD. From the right parasternal long-axis view, the SVC and IVC are aligned and then a rightward sweep may identify a defect at the entry of the SVC or IVC.
RT3D TTE: Parasternal short axis and apical and subcostal four-chamber views. Also, right parasternal approach with the patient placed in the right lateral decubitus position.
Two-dimensional TEE midesophageal four-chamber view allows optimal visualization of the entire interatrial septum, the atrioventricular valves, and the subvalvular apparatus. From this view, a careful multiplane interrogation of the entire septum allows for identification and characterization of all types of ASD.
TEE four-chamber view with clockwise rotation to focus on the RA and with the multiplane sector scan at 60 to 90 degrees to assess the atrial septum, IVC (on the left), and SVC (on the right) to identify sinus venosus defects.
RT3D TEE midesophageal at 0 (four-chamber view) and 90 degrees (usually a bicaval view) (9):
From the 0-degree 2D view and using the 3D zoom feature, tilt the image up for en face visualization of the interatrial septum from the RA. Then, tilt the image left for en face visualization of the interatrial septum from the LA.
From a 90-degree 2D image, tilt the image up and then rotate it 90 degrees to the left on the z-axis.
ICE longitudinal view to assess the atrial septum from cranial to distal margins and the perpendicular short-axis view to assess the anterior septum and its transition to the ascending aorta. ICE is best suited for detection of secundum ASD and PFO (10).
Key Diagnostic Features
TTE or TEE M-mode is limited in the diagnosis of ASD but provides information on right ventricle (RV) volume or pressure overload, or both; hypertrophy; and dilatation:
In volume overload, the septum is displaced posteriorly or toward the left ventricle (LV) during mid- to late diastole.
In pressure overload, which follows volume overload, the septum is displaced posteriorly during late systole and early diastole (see Fig. 5.1).
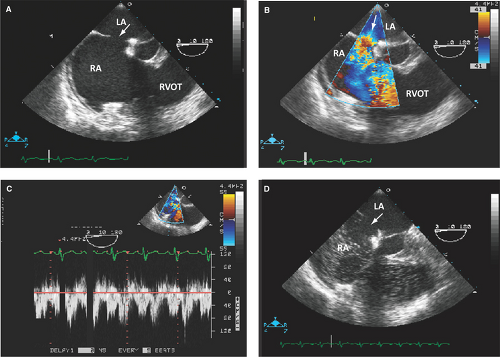
By 2D TTE, TEE, or ICE, and confirmed on surgery, an ASD appears as an area of focal discontinuity in the interatrial septum with sometimes broadening of the edges of the defect (2,3,6,7,10) (Figs. 12.1A; 12.2; 12.3A,B; 12.4; and 12.5A).
Atrial septal tissue of variable extent above the base of the atrioventricular valves suggests a secundum ASD (Fig. 12.1A).
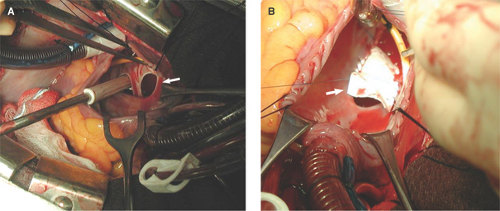
Figure 12.2: These surgical views from a right atriotomy in the patient described in Figure 12.1 demonstrate a large ASD (arrow in A) and a partially patched ASD (arrow in B).
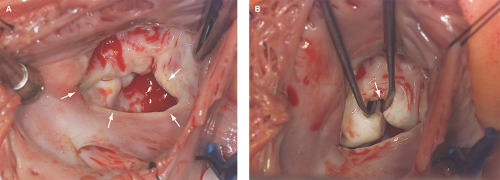
Figure 12.4: These surgical views from a right atriotomy in the patient described in Figure 12.3 demonstrate a large ostium primum ASD (arrows in A) and a cleft anterior mitral leaflet (arrow in B).
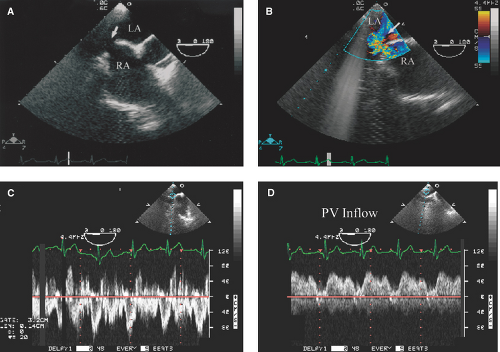
Absence of septal tissue in the most anterior portion of the interatrial septum (at the level of the atrioventricular valves) is diagnostic of an ostium primum defect (Figs. 12.3A,B and 12.4A):
An ostium primum ASD is associated with a cleft anterior mitral leaflet (Fig. 12.4B).
Also in these patients, chordal attachments of the anterior leaflet to the interventricular septum causing LV outflow obstruction can be seen.
Two-dimensional TEE or ICE determines location and diameter of an ASD and its relation to adjacent heart valves and veins, and defines surrounding tissue availability for appropriate transcatheter closure device position and deployment (3,6,10).
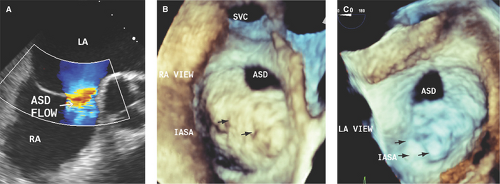
Two-dimensional TEE and ICE are more accurate than TTE in the detection of sinus venosus ASD and associated anomalous pulmonary venous return (100% with sinus venosus ASD and 9% with secundum ASD) (Fig. 12.5):
In these patients, RV dilatation out of proportion to the size of the ASD suggests anomalous pulmonary venous return.
Two-dimensional TEE and ICE are more accurate than TTE in defining the size of an ASD as PFO (<5 mm diameter with right-to-left shunting), small ASD (5 to 10 mm in diameter), moderate ASD (11 to 20 mm), or large ASD (>21 mm) (Figs. 12.1 through 12.6).
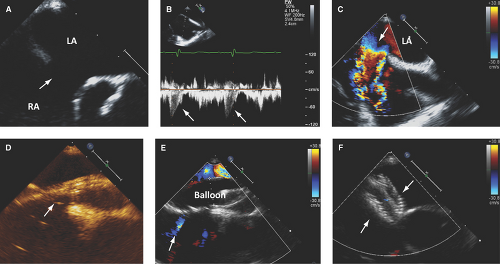
Two-dimensional TEE or ICE helps guiding transcatheter closure device positioning and deployment (Fig. 12.6):
These techniques can detect the presence and degree of residual shunting and need for device reposition or redeployment (3,6,7,10,11).
Are safe (<1% complication rate), contribute to a high (up to 98%) success rate of transcatheter closures, and minimize patients’ exposure to radiation.
Can detect surgical periprocedural complications (i.e., closure of the os of the coronary sinus or part of the IVC instead of the septal defect).
Are accurate in the detection of rarely associated atrial thrombi (incidence of 1.2% in ASD and 2.5% in PFO) and up to 3% after transcatheter closure of ASD or PFO (70% are detected within 4 weeks of device deployment) (12).
RT3D TTE and TEE are feasible in >95% of patients with ASD and are recommended techniques in the diagnosis, characterization, and guidance during closure of ASD (4,5,8,9,13,14,15,16,17).
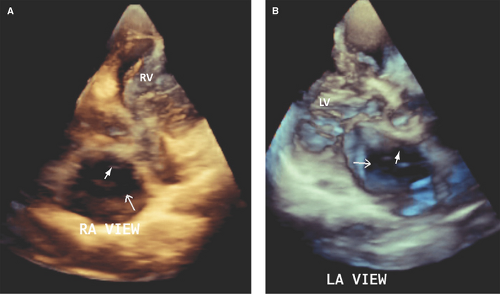
RT3D TTE and especially TEE are superior to 2D TTE, TEE, and ICE in determining the type, shape (often oval or irregular, uncommonly fenestrated), true largest diameter (best assessed during ventricular systole), and area of ASD (4,8,9,14,15,16,17) (Figs. 12.7 and 12.8).
RT3D TEE accurately defines the extent of atrial septal tissue surrounding an ASD:
The atrial septal tissue rims of an ASD are classified as superoanterior (tissue rim from the defect to the aortic annulus), inferoanterior (rim from the defect to atrioventricular valves), superoposterior (rim from the defect to the SVC), and inferoposterior (rim from the defect to the IVC).
Accurate assessment of the size of the ASD and surrounding extent of tissue rims is essential in determining the suitability of an ASD for percutaneous device closure.
Percutaneous closure of a secundum ASD is feasible if the largest diameter of the defect is <35 mm, its superoanterior or aortic tissue rim is >3 to 5 mm, and other rims are >7 mm (Figs. 12.7 and 12.8).
RT3D TEE imaging also provides clear visualization and guidance on the placement of the guide wire and sheath across the ASD and allows accurate sizing of a closure device:
The size of a closure device is determined when an expanded sizing balloon ceases flow by color Doppler (called stop-flow diameter).
The final size of a closure device is 1 to 2 mm larger than the stop-flow diameter.
RT3D TEE is also accurate in determining the proper placement of the closure device:
It determines if sufficient tissue rim is caught between the two plates of the device.
Identifies device closure malpositioning and provides guidance for its repositioning.
Saline Contrast Echocardiography
Key Diagnostic Features
Shunting in ASD is predominantly left to right, but most defects have right-to-left or bidirectional
shunting. Thus, saline contrast echo is sensitive in detecting ASD or PFO.
Bubbles enter the LA during early systole, inspiration, Valsalva maneuver, or coughing (RA pressure is transiently higher than LA pressure) and within three cardiac cycles of their appearance in the RA (more than four cardiac cycles suggests a pulmonary arteriovenous fistula).
The predominant left-to-right shunting is seen as a negative contrast effect or washout of bubbles in the RA side of the septum (Figs. 12.1D and 12.3D).
Assessment of the Severity of Atrial Septal Defects with Doppler Echocardiography
Pulsed Wave Doppler: Diagnostic Methods and Key Diagnostic Features
Pulsed wave Doppler and 2D echo are used to estimate the ratio of right heart or pulmonary (Qp) to left heart or systemic (Qs) stroke volume as follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree