Fig. 11.1
Acute intracerebral hemorrhage (ICH), appearing as a hyperdense (bright) lesion on computed tomography (CT) scan
In some cases the blood may extend into the ventricles or, less commonly, the subarachnoid space. The annual incidence is 10–30 per 100,000 population, accounting for two million (10–15 %) of about 15 million strokes that occur worldwide each year. Although the age-specific incidence of ICH is stable or decreasing, probably due to better population control of hypertension, the absolute number of ICHs is expected to rise in the next decades because of aging of the population. Compared to ischemic stroke, the case fatality rate of ICH is much higher, with only 38 % of affected patients surviving the first year. Many survivors are left with significant residual disability.
Depending on the underlying cause of bleeding, ICH is classified as either primary or secondary [1]. Primary ICH, accounting for 78–88 % of cases, originates from the spontaneous rupture of small vessels damaged by chronic hypertension or cerebral amyloid angiopathy (CAA). Secondary ICH occurs in a minority of patients in association with vascular abnormalities (such as arteriovenous malformations and aneurysms), tumors, or impaired coagulation. However, ICH associated with anticoagulant medication is typically considered a form of primary ICH; for example, evidence suggests that warfarin-related ICH is associated with the same small vessel diseases, such as CAA, that cause primary ICH [2]. There are many potential causes of secondary ICH (see Table 11.1). Except for vascular malformations as a cause of ICH in younger patients, these secondary causes are rare. Younger patients with ICH should have angiography to rule out secondary causes.
Table 11.1
Primary and secondary causes of intracerebral hemorrhage (ICH)
Primary ICH |
Hypertensive arteriolosclerosis |
Age-related arteriolosclerosis |
Cerebral amyloid angiopathy |
Secondary causes of ICH |
Vascular malformations |
Arteriovenous malformation |
AV dural fistula |
Cavernous hemangioma |
Hemorrhagic transformation of ischemic stroke |
Related to arterial infarction |
Related to venous infarction |
Vasculitis |
Moyamoya disease |
Coagulopathy |
Related to anticoagulant use |
Related to thrombolytic use |
Thrombocytopenia |
Decreased synthesis of clotting factors (e.g., hemophilia, liver disease) |
Increased consumption of clotting factors (e.g., disseminated intrasvacular coagulation) |
Brain tumor |
Aneurysm |
Ruptured saccular aneurysm |
Ruptured mycotic aneurysm |
Related to sympathomimetic drug use |
Amphetamines |
Cocaine |
Phenylpropanolamine |
Ephedrine |
Trauma |
The most common locations of ICH are the cerebral lobes (35 %), the basal ganglia (30 %), the thalamus (20 %), the brainstem (5 %), and the cerebellum (10 %) (Fig. 11.2) [3]. It is useful to discriminate between lobar and non-lobar locations of primary ICH, because the associated causes and risk factors differ by location. Lobar ICH may be caused by either CAA or hypertensive arteriosclerosis, while non-lobar primary ICH is almost exclusively caused by hypertensive arteriosclerosis. This is because CAA affects the cortical and leptomeningeal arteries but not the arteries penetrating into the substance of the brain, while most hypertension-related ICHs occur at or near the bifurcation of small penetrating arteries that originate from the major branches of the circle of Willis. Accordingly, hypertension is a stronger risk factor for non-lobar ICH than lobar ICH, while conversely the presence of one or more apolipoprotein E epsilon 4 (apoE ε4) alleles, associated with the presence of CAA, is a stronger risk factor for lobar ICH than non-lobar ICH [4, 5]. Other risk factors for ICH include age, male sex, cigarette smoking, low (not high) serum cholesterol levels, and Hispanic, African, or Asian racial origin [1, 6].
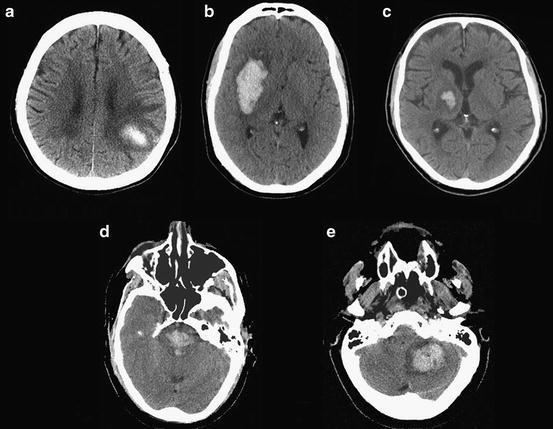

Fig. 11.2
Common locations of ICH: (a) cerebral lobe (lobar), (b) basal ganglia (putamen), (c) thalamus, (d) brainstem, (e) cerebellum
Cognitive Symptoms and Signs in Acute ICH
Cognitive symptoms in acute ICH depend on the location of bleeding. ICH may cause neurological dysfunction by local destruction of tissue, local mass effect with compression of tissue, potential toxic effects of iron or other molecules found in coagulating blood, or increased intracranial pressure caused by mass effect or obstruction of the ventricular system.
Small- to medium-size ICHs that do not increase intracranial pressure typically present with focal neurological symptoms reflecting the site of origin of the ICH. These may include hemiparesis, hemiplegia, aphasia (resulting from ICH in the left perisylvian cortex), or neglect or visual–spatial difficulty (most commonly resulting from ICH in the parietal cortex). ICH in the thalamus may also present with these “cortical” signs of aphasia (left thalamus) or neglect (right thalamus). ICH in the basal ganglia is usually associated with more prominent motor signs, such as hemiparesis, than cognitive signs; however, survivors of basal ganglia ICH often have cognitive deficits as well (discussed in the subsequent section: “Cognitive Consequences of ICH”).
Larger ICHs that cause increased intracranial pressure, or ICHs in the brainstem or thalamus that affect the reticular activating system or its connections in the thalamus, often present with depressed level of consciousness in addition to other signs. In many cases the level of consciousness is significantly depressed and confounds the ability to assess other aspects of cognition. Depressed level of consciousness at presentation is more common in ICH than ischemic stroke; however, there are no signs or symptoms that reliably distinguish between ICH and ischemic stroke.
Delirium is a frequent complication in the first several weeks following ICH. Many factors may contribute to the risk of delirium; among them are the size of the ICH and the presence of intraventricular hemorrhage. Neurological toxic effects of blood break-down products may also contribute. In animal studies, experimental ICH is followed by extensive brain inflammation that worsens cognitive and motor function. The significance of post-ICH secondary injury in humans remains unclear, although post-ICH edema can be detected using neuroimaging. Patients with ICH have as much as a fivefold increased odds of post-stroke cognitive impairment and delirium in the first few weeks compared to patients with ischemic stroke [7].
Cognitive Consequences of ICH
ICH is the most severe form of stroke. Most survivors will have residual disabilities, and cognitive symptoms are common.
Dementia frequently accompanies stroke, both ischemic and hemorrhagic. According to a recent systematic review, about one in ten patients have dementia before their first stroke and one in ten develop new dementia after their first stroke. The risk of post-stroke dementia is higher in ICH than in ischemic stroke, reflecting the greater severity of ICH [8]. Risk factors for dementia following ICH specifically, as opposed to stroke in general, have not been well defined. Based on studies done in consecutive patients with either ischemic stroke or ICH, the expected risk factors for dementia post-ICH might be higher initial stroke severity, advanced age, left hemisphere origin, aphasia, and prior history of stroke or dementia [8].
A history of dementia preceding ICH is seen in 15–23 % of patients [9, 10]. Preexisting dementia is associated with increasing age, female gender, low educational level, severity of cerebral or temporal lobe atrophy, previous stroke or transient ischemic attack, severity of leukoaraiosis, diabetes, atrial fibrillation, and arterial hypertension. The high prevalence of pre-ICH dementia suggests that dementia may be more common in persons who develop ICH than other similar-aged persons, although this has not been proven in an epidemiologic study. Patients who develop ICH could be at risk for dementia, independent of the ICH, because of shared risk factors. Hypertensive arteriolosclerosis and CAA, the most common causes of ICH, also cause brain microscopic infarction and ischemic white matter demyelination (also known as leukoaraiosis) that contribute to the risk of dementia. Leukoaraiosis is frequently seen in the brains of persons with ICH and is associated with pre-ICH dementia (Fig. 11.3) [10, 11]. CAA may be accompanied by Alzheimer’s pathology, although fewer than half meet pathological criteria for Alzheimer’s disease [12].
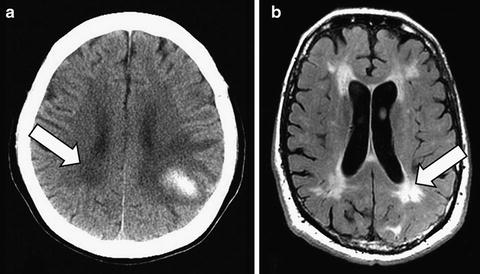

Fig. 11.3
Leukoaraiosis appears on CT as periventricular white matter hypodensities in this patient with a left parietal lobar ICH (a, arrow), and on T2-weighted MRI as hyperintensities (different patient) (b, fluid attenuated inversion recovery [FLAIR] sequence, arrow)
Milder forms of cognitive impairment are even more common than dementia following stroke [13]. As a screening test for cognitive impairment, the Montreal Cognitive Assessment tool (MoCA) is more sensitive at detecting milder forms of cognitive impairment than the Mini-Mental State Examination (MMSE) [14]. However, there are very limited data on the prevalence of mild cognitive impairment following ICH specifically.
The pattern of deficits on neuropsychological testing in ICH patients depends on the location and severity of the hemorrhage, the ability of the brain to accommodate the cerebral damage (which in turn depends on the overall health of the brain, including the degree to which the brain is damaged by other effects of small vessel disease such as leukoaraiosis), and the degree of neurological recovery. Therefore, the resulting neuropsychological syndrome may reflect a combination of focal deficits related to the site of origin of the ICH, as well as global deficits as a consequence of more widespread neuronal injury caused by globally raised intracranial pressure, for example. Although it is useful to consider the neuropsychological deficits from ICH in terms of site of origin, it also must be recognized that the ICH may have extended from the site of origin to involve neighboring white matter or other brain structures.
Patients with lobar brain hemorrhages are expected to display abnormalities on neuropsychological testing that reflect a decline in function in the areas affected by the hemorrhage. For example, a patient with a left posterior superior temporal ICH may show symptoms of Wernicke’s aphasia.
Patients with the more common non-lobar brain hemorrhages may display a variety of deficits. Non-lobar hemorrhages most commonly affect subcortical brain regions—the putamen, head of the caudate nucleus, and thalamus—that communicate widely with the frontal lobes via feedback loops to regulate thought and mood.
The striatum (caudate nucleus and putamen) is the main input structure of the basal ganglia. Given the connections between the striatum and the convergent loops related to the major cortical areas of the brain, damage to the striatum may result in a multitude of deficits pertaining to motor, oculomotor, cognitive, associative, and limbic functions. Indeed, ICH in the basal ganglia has been associated with poor performance in multiple cognitive domains including language, attention, memory, visuospatial processing, and executive function abilities, with many patients showing impairment in multiple domains [15].
Patients with ICH in the putamen may have impairments in executive function, visuospatial function (including neglect), apraxia, and aphasia. On the Wisconsin Card Sorting Test (WCST), patients with putaminal ICH have been shown to have impaired mental set shifting with disrupted concept formation or problem solving (the inability to comprehend the possible sorting rules) and perseveration (the inability to flexibly shift attention and response preparation from one set of rules to another) [16]. Unilateral spatial neglect can be detected in up to half of patients [17, 18]. Motor apraxias may occur in 20–40 % [19]. Aphasia may occur, and is related, at least in part, to extension of the hemorrhage into adjacent white matter pathways [20]. Extension into the deep frontal and anterior periventricular white matter causes decreased fluency, while extension to the deep temporal white matter causes impaired word comprehension. Hemorrhage extension into both the anterior and posterior periventricular white matter may cause a global aphasia. Extension more laterally into the region of the external capsule may cause conduction aphasia [21, 22]. Buccofacial oral apraxia often accompanies the aphasia [23].
Patients with ICH in the head of the caudate nucleus may have impairments in executive function, short-term and long-term memory, apathy, or aphasia [24–26]. Impaired mental set shifting and perseveration may be seen on the WCST [24]. One study suggests that the most prominent impairments are in short-term memory, long-term memory, and verbal fluency [24]. Aphasia following hemorrhage restricted to the caudate is rare, but is not infrequent when the hemorrhage also involves the neighboring white matter tracts [26].
Patients with ICH in the thalamus may have impairments in executive function, short-term and long-term memory, apraxia, aphasia, or neglect. Thalamic aphasia may be seen in lesions located in the dominant hemisphere and is characterized by fluent speech with paraphasias, perseveration, and lack of spontaneous speech but relatively preserved comprehension and repetition. Unilateral spatial neglect is more common with right-sided lesions, and often improves clinically over time [27].
In sum, a wide variety of neuropsychological deficits may be detected in survivors of ICH. Patients with small to moderate-sized lobar ICHs may have focal neuropsychological deficits related to the site of injury, while patients with larger ICHs or ICHs originating in non-lobar subcortical structures typically have deficits in multiple domains, including aspects of executive function, visuospatial function, aphasia (for dominant hemisphere lesions), and neglect (for nondominant hemisphere lesions). In patients with subcortical ICHs, particularly those originating in the putamen and caudate nucleus, the cognitive deficits may predominantly reflect injury to adjacent structures including white matter pathways, rather than damage to the putamen or caudate themselves. The cognitive profiles of ICH are not distinct enough to discriminate ICH from ischemic stroke based on neuropsychological testing alone, although patients with ICH have been described to have somewhat more prominent problems with higher-level perceptual functions [25].
Diagnosis and Medical Management
There are no signs or symptoms that reliably distinguish between ICH and ischemic stroke; therefore, neuroimaging is needed to distinguish between the two [28]. Computed tomography (CT) shows similar sensitivity to magnetic resonance imaging (MRI) for acute hemorrhage and is often the initial investigation of choice. Medical management includes supportive care, control of excessively high blood pressure, lowering intracranial pressure if necessary, provision of hydration and nutrition, and prevention and treatment of medical complications such as seizure, deep venous thrombosis, and infection [28]. Severely affected patients may require intubation and mechanical ventilation. Limitation of care or palliation may be appropriate in severely affected patients with little hope of survival or meaningful quality of life [29, 30]. Surgical removal of the hematoma is often performed for patients with secondary ICH, cerebellar ICH, or with lobar ICH and clinical deterioration, and less commonly for patients with non-lobar-hypertensive ICH [31]. Expansion of the ICH occurs in up to 40 % of patients within 24 h of hospital presentation and may be lessened by good blood pressure control [32]. Trials of hemostatic therapies have not shown a clinical benefit despite demonstration of a modest reduction in ICH growth [33].
The risk of recurrent hemorrhage is approximately 2 % per year for non-lobar ICH and 5–10 % per year for lobar ICH. Recurrences are more frequent when blood pressure is poorly controlled. Among lobar ICH patients, risk factors for recurrence include prior symptomatic ICH, a greater number of MRI-defined microbleeds, the presence of one or more apoE ε(epsilon)2 or ε(epsilon)4 alleles, and the probable presence of underlying CAA. Risk factors for recurrence in patients with proven CAA are the same but also include the use of aspirin and the presence of leukoaraiosis [10, 34, 35].
Rational steps to prevent recurrence include blood pressure reduction and cessation of smoking and alcohol use [28]. However, these secondary prevention strategies are of unproven benefit with the exception that data from a randomized trial support an effect of blood pressure reduction [36, 37].
Whether better supportive care leads to improved cognitive outcomes is currently not known. There are no proven therapies for cognitive rehabilitation that are specific to ICH. Therefore, cognitive rehabilitation in ICH should follow the same principles as for ischemic stroke. This may include pharmacotherapy for depression or apathy. In our experience, stimulants such as methylphenidate may be used in ICH patients provided that blood pressure is monitored and controlled to avoid the risk of hypertension.
Brain Microbleeds
Microbleeds represent small (usually less than 5 mm) prior asymptomatic brain hemorrhages. Histopathologically, they appear as areas of perivascular hemosiderin deposition [38]. The hemosiderin molecule contains iron atoms derived from the breakdown of hemoglobin from red blood cells that previously leaked into the tissue. After deposition, the hemosiderin molecules remain in location indefinitely. Therefore the hemosiderin deposits across the brain represent the cumulative distribution of all the small hemorrhages experienced during the patient’s life. These hemosiderin deposits are visible pathologically but may be overlooked because of their small size (usually 5 mm or less, and often as small as 1–2 mm). The deposits cannot be visualized on CT or on conventional MRI sequences but can be seen as “microbleeds” on specialized MRI sequences—T2*-weighted gradient-recalled echo (GRE) or susceptibility-weighted imaging (SWI)—where they appear as small dark round (i.e., hypointense) foci [39] (Fig. 11.4).
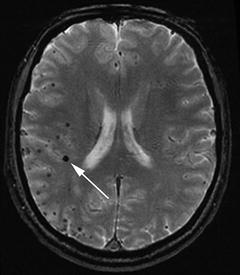

Fig. 11.4
Microbleeds, evident as small black dots on MRI T2*-weighted gradient-recalled echo (GRE) sequence. The white arrow points to the largest microbleed
Microbleeds are a common finding in asymptomatic elderly, being present in at least 5–10 % of persons over the age of 60 [40]. However, the prevalence is much higher in persons with ischemic stroke (20–30 %) or ICHs (50–70 %). Microbleeds are a sign of cerebral small vessel disease, and when seen in large numbers, are most commonly associated with either poorly controlled hypertension or CAA. When multiple and restricted to the cerebral cortex or subcortical junction, without involvement of subcortical structures such as the basal ganglia, they are highly suggestive of the pathological presence of CAA (see also the subsequent section: “Cerebral Amyloid Angiopathy”) [41].
Previously, microbleeds were considered too small to directly affect brain function. More recently, this assumption has been questioned because of emerging evidence that microbleeds may be associated with cognitive impairment. For example, a case–control study done in patients seen in a neurovascular clinic found that executive dysfunction was more frequent in 25 patients with microbleeds compared to 30 controls without microbleeds, matched for stroke type, stroke location, and the extent of MRI white matter lesions [42]. In this study, executive dysfunction was defined as poor performance on two or more of the following tests: word fluency, Stroop, trail making, WCST, or Weigl Sorting Task. The stroke patients with executive dysfunction were more likely to have microbleeds in the frontal lobes and basal ganglia [42]. Microbleeds are frequently seen in persons with vascular dementia, but are also seen in up to 20 % of patients with mild cognitive impairment or clinically probable Alzheimer’s disease [43–50]. Microbleeds have been associated with worse cognitive performance in adults without diagnosed neurological diseases [51–53].
However, caution is warranted before ascribing cognitive symptoms to the direct effects of microbleeds. Microbleeds may be merely a sign of an underlying cerebral small vessel disease causing cognitive dysfunction by mechanisms other than bleeding. For example, both hypertensive arteriopathy and CAA are associated with microbleeds but also cause microscopic infarctions that, in contrast to microbleeds, cannot be detected on MRI. Further studies are needed to clarify whether microbleeds affect brain function directly, or are merely an associated finding.
Cerebral Amyloid Angiopathy
CAA is caused by deposition of amyloid, a protein aggregate, in the media and adventitia of small arteries of the brain [54]. In most cases the amyloid is beta-amyloid, derived from the abeta protein. In some rare autosomal dominant hereditary diseases, the amyloid is derived from other proteins including transthyretin (in transthyretin amyloidosis), cystatin C (Icelandic familial dementia), or BRI (British familial dementia) [55]. CAA is most commonly clinically recognized as a cause of approximately half of lobar ICHs. However, the spectrum of clinical presentations of CAA also includes small sulcal subarachnoid hemorrhages, vasculitis, and transient neurological symptoms, and there is increasing evidence that CAA is a frequent contributor to the risk of dementia.
Arteries affected by amyloid deposition have thickened walls, fibrin deposition within the wall, and loss of wall integrity including circumferential cracking (a “vessel within a vessel” appearance) [54]. There may be perivascular hemosiderin deposits indicating leakage of red blood cells through the arterial wall [56]. Pathologically, the presence of amyloid may be diagnosed by the characteristic apple-green colored birefringence seen when affected arteries are stained with Congo red and then viewed under polarized light. An alternate means of pathological diagnosis is to identify the amyloid protein using an immunohistochemical stain.
Sporadic non-inherited CAA caused by beta-amyloid is a common age-associated pathology [57]. As with Alzheimer’s disease, the prevalence of CAA is highly age-dependent and increases exponentially with increasing age. Some degree of CAA is seen in more than 40 % of persons over 80 [58]. In many cases, affected persons appear asymptomatic, although increasing evidence suggests that CAA is an important under-recognized contributor to the risk of dementia.
The common sporadic CAA is caused by aggregation of abeta into beta-amyloid with deposition in the vascular media. Abeta is derived from proteolytic processing of the amyloid precursor protein by beta-secretase and gamma-secretase. After formation, abeta can have several fates including aggregation into beta-amyloid with deposition in the arterial wall or brain parenchyma, proteolytic cleavage by enzymes such as neprilysin or insulin-degrading enzyme, or clearance from the brain via a perivascular interstitial fluid drainage pathway [59]. Aggregation of abeta with deposition as beta-amyloid may result from an imbalance between production and clearance of abeta [60]. Vascular beta-amyloid deposits are almost exclusively restricted to the leptomeningeal and cortical arteries.
The beta-amyloid seen in CAA is the same beta-amyloid that is the main constituent of the neuritic plaques seen in Alzheimer’s disease. Indeed, the frequency and severity of Alzheimer’s pathology is higher in the brains of persons with CAA than persons without [12]. CAA and Alzheimer’s pathology may be conceived as being situated on the opposite ends of a spectrum, with some patients predominantly exhibiting vascular amyloid deposition (i.e., CAA) with increased risk of lobar ICH, and other patients predominantly exhibiting parenchymal amyloid deposition (i.e., Alzheimer’s pathology) with increased risk of Alzheimer’s type dementia.
The cause of nonhereditary beta-amyloid CAA is unknown. Other than age, the only other known risk factor is the presence of one or more apoE ε(epsilon)4 alleles [12]. Patients with one or more apoE ε(epsilon)2 alleles have more severe vasculopathic changes and may be at increased risk for ICH [61, 62]. Lowering of blood pressure and avoidance of antithrombotics and anticoagulants are reasonable steps to reduce the risk of recurrence.
Clinically, CAA is best recognized as a cause of approximately half of lobar ICHs in developed countries where there is good treatment of chronic hypertension. The hemorrhages may occur in any brain lobe, but have a predilection for posterior brain regions including the occipital lobe and posterior temporal and parietal lobes [63]. In about half of the cases of pathologically proven CAA-associated ICH, the lobar ICH is accompanied by one or more lobar microbleeds (Fig. 11.5) [41]. The presence of microbleeds in other locations not involved with vascular amyloid, such as the thalamus, basal ganglia, or brainstem, is not consistent with CAA and should prompt consideration of other causes such as hypertensive arteriolosclerosis. Diagnostic criteria for clinical research, termed the Boston criteria, discriminate between probable and possible CAA based on presentation with lobar ICH and the presence or absence of accompanying lobar hemorrhages or microbleeds, without identifiable alternate causes (Table 11.2) [41]. Positron emission tomography (PET) with Pittsburgh Compound B or other beta-amyloid ligands will identify vascular beta-amyloid as well as parenchymal beta-amyloid, and could therefore be useful to confirm beta-amyloid deposition [64].
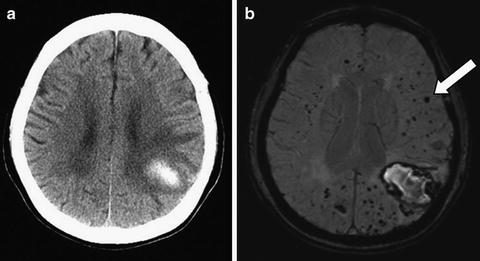

Fig. 11.5
Cerebral amyloid angiopathy (CAA). In this 70-year-old woman with left parietal lobar ICH (a, CT scan), MRI susceptibility-weighted imaging reveals dozens of small hypointensities indicating lobar microbleeds (b, arrow) not apparent on CT scan, consistent with probable CAA
Table 11.2
Boston criteria for diagnosis of cerebral amyloid angiopathy (CAA)-related ICH
Definite CAA | Full postmortem examination showing severe CAA |
|---|---|
Probable CAA with supporting pathology | Pathologic tissue (evacuated hematoma or cortical biopsy) showing |
Lobar ICH with some degree of CAA | |
Absence of other diagnostic lesion | |
Probable CAA | Clinical data and MRI or CT demonstrating |
Multiple hemorrhages restricted to lobar regions or a single lobar hemorrhage with additional evidence of superficial siderosis | |
Age ≥55 years | |
Absence of other cause of hemorrhage or superficial siderosis | |
Possible CAA | Clinical data and MRI or CT demonstrating |
Single lobar hemorrhage, or focal or disseminated superficial siderosis | |
Age ≥55 years | |
Absence of other cause of hemorrhage or superficial siderosis |
There are several less common presentations of CAA. Some patients present with vasculitis or perivascular inflammation and signs of patchy focal or multifocal edema in the periventricular white matter or juxta-cortical white matter, often involving the temporal or parietal lobes [66]. These patients may have a subacute cognitive decline, headaches, seizures, or focal neurological deficits such as hemiparesis. In some cases, the clinical course may be that of a rapidly progressive dementia. Neuroimaging shows brain edema, usually without infarction. In contrast to chronic white matter lesions, which are also common in CAA, the white matter lesions seen in CAA-associated vasculitis are not associated with atrophy but rather exhibit swelling and mass effect. When the edema is very focal, it may be mistaken for a low-grade tumor. There are usually multiple lobar microbleeds, although we have also seen pathologically proven cases without microbleeds. The cerebrospinal fluid often exhibits increased protein and sometimes a lymphocytic pleocytosis, but may also be normal. The diagnosis of CAA with vasculitis may be suspected on clinical and radiological grounds, but can only be definitively proven by biopsy. Immunosuppressive therapy, for example with steroids, can produce a dramatic clinical and radiological improvement in up to two-thirds of patients. Responders may stay in remission for years without need for chronic immunosuppression [66].
CAA is an important contributor to the risk of dementia, even in the absence of lobar ICH and accounting for the effects of accompanying Alzheimer’s pathology. In the population-based Medical Research Council-Cognitive Function and Aging Study (MRC-CFAS) autopsy study, CAA accounted for 7 % of the risk of dementia, controlling for Alzheimer’s disease pathology [67]. Similarly, another population-based study showed that for a given severity of Alzheimer’s pathology, a higher degree of CAA was associated with worse performance on a global cognitive test [68].
The cognitive impairment seen in cerebral amyloid-angiopathy that is independent of hemorrhagic stroke is probably caused by brain ischemia. CAA is associated with a high burden of periventricular white matter demyelination [10], presumably on an ischemic basis, and small cerebral infarctions. Disturbed blood flow regulation with impaired vasodilation might play a role in generating these small infarctions [57].
There is limited information on the pattern of cognitive deficits that can be attributed to CAA, independent of the effects of lobar ICHs and coexisting Alzheimer’s pathology. Based on the association between CAA and subcortical white matter lesions, deficits may be expected to include those associated with white matter disease, including poor performance on timed tests. However, cortical microscopic infarctions and microbleeds could produce signs of cortical dysfunction dependent on the location and severity of the pathological changes. A prospective cohort study with brain autopsy found that more severe CAA was correlated with worse performance on the symbol digit modalities test and a test of episodic memory, controlling for the amount of Alzheimer’s pathology and in the absence of ICHs [69]. This poor performance on a timed test influenced by visual perception may reflect a combination of the subcortical ischemic white matter demyelination and predilection for the posterior parietal lobe and occipital lobe that are seen in cerebral amyoid angiopathy.
In summary, three potential contributing factors must be considered when evaluating cognition in a patient with probable CAA: (1) the effects of lobar ICH, if one has occurred; (2) the effects of accompanying Alzheimer’s pathology, which may or may not be present; and (3) the effects of CAA independent of either lobar ICH or accompanying Alzheimer’s pathology, which are presumably mediated by brain ischemia. The neuropsychological evaluation should therefore include tests sensitive to the cortical deficits expected based on the lobar ICH location, tests sensitive to the pattern of impaired memory retrieval seen in Alzheimer’s disease, and tests sensitive to the impairments in psychomotor processing speed and executive dysfunction that may be seen with subcortical ischemic white matter disease.
Other Less Common Causes of Ischemic Stroke
The common causes of ischemic stroke are arterial disease related to hypertension and atherosclerosis, or cardiac disease related to coronary heart disease or atrial fibrillation. A substantial number of ischemic strokes are cryptogenic, which is defined as having no diagnosable cause. Other determined causes of stroke are rare. A comprehensive discussion of all uncommon causes of stroke is outside the scope of this chapter. Instead, we will highlight selected less common causes of stroke where cognitive impairment may be a prominent feature.
Cerebral Autosomal Dominant Arteriopathy with Subcortical Ischemic Leukoencephalopathy
Cerebral autosomal dominant arteriopathy with subcortical ischemic leukoencephalopathy (CADASIL) is a rare genetic cause of stroke, migraine, and vascular dementia [70]. The disease is caused by mutations of the NOTCH3 gene, which encodes the Notch3 receptor. Small arteries become thickened and fibrosed. Electron microscopy shows granular osmiophilic material adjacent to the vascular basement membrane, which is pathognomonic for the disease. Symptoms are limited to the central nervous system (CNS), even though arteries outside the brain are affected, in addition to those within the brain. Neuroimaging reveals multiple lacunar infarcts in the basal ganglia, thalamus, and white matter, along with extensive white matter demyelination (Fig. 11.6). Typically, the disease begins with migraine with aura in the victim’s late 20s, lacunar strokes occur in their 40s, and vascular cognitive impairment and dementia occur in their 40s and 50s [71]. The diagnosis can be made by genetic analysis, or by skin or muscle biopsy with electron microscopy to identify the characteristic vascular changes with granular osmiophilic material.
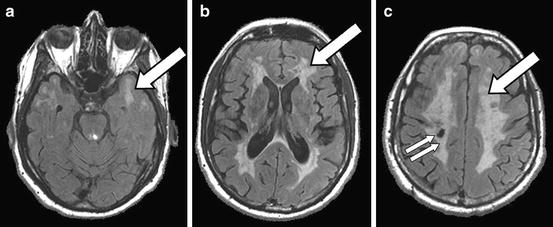

Fig. 11.6
Cerebral autosomal dominant arteriopathy with subcortical ischemic leukoencephalopathy (CADASIL): a 41-year-old man with CADASIL confirmed by genetic testing. The MRI FLAIR sequence shows extensive white matter T2-hyperintensities (a–c, arrows), including hyperintensities in the anterior temporal white matter (a) that are characteristic of CADASIL compared to other white matter diseases, and a lacunar infarct in the right corona radiate (c, double arrow)
CADASIL is an instructive disease because it is the prototypical example of a pure subcortical ischemic dementia. Because CADASIL patients develop dementia at an early age, the cognitive profile of CADASIL is uncomplicated by other late-life pathologies of dementia such as Alzheimer’s disease. In the earlier stages, CADASIL patients may display poor performance on neuropsychological tests sensitive to executive dysfunction including the Trail Making Test and tests of verbal fluency [72, 73]. As the disease progresses, more global cognitive impairments are seen, including memory. Apathy is a prominent neuropsychiatric symptom [74]. A randomized placebo-controlled trial of the acetylcholinesterase inhibitor donepezil showed that treated patients had modest improvements on the Trail Making Test but no improvement in the global cognitive endpoint compared to the placebo group [75].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







