Key Clinical Questions
What findings are commonly seen on physical examination, laboratory studies, and imaging studies in patients with cirrhosis?
How is the etiology of ascites determined and by sending what studies of ascitic fluid?
What are common causes of renal failure in patients with cirrhosis?
How is hepatorenal syndrome diagnosed and treated?
How can hepatic encephalopathy present clinically, and what are the treatment options?
Introduction
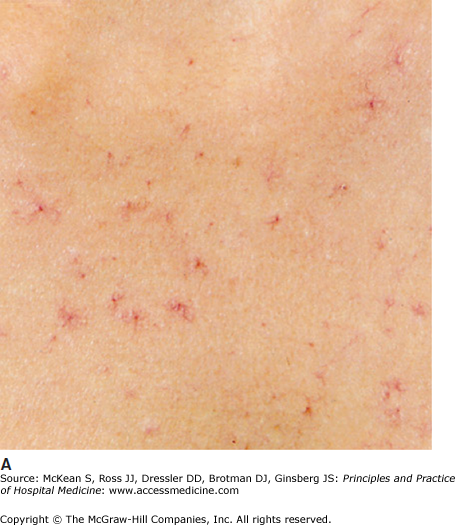
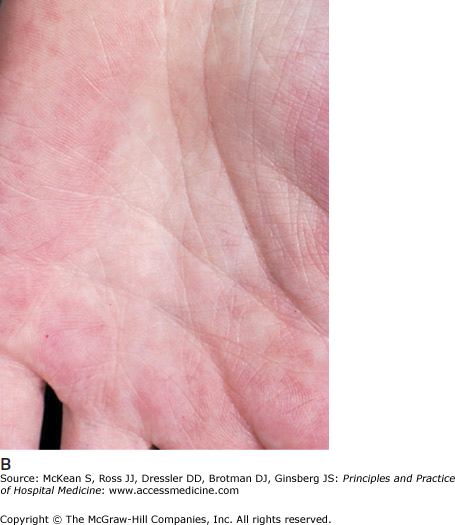
Cirrhosis describes the end stage of chronic liver disease from any etiology. Histologically, injured and regenerating hepatocytes are surrounded by extracellular matrix or fibrosis, creating a nodular appearance to the liver parenchyma. A presumptive diagnosis of cirrhosis without histologic confirmation can often be made on clinical grounds alone. A palpable, enlarged left liver lobe with a small right lobe is highly suggestive of cirrhosis. Splenomegaly implies the presence of portal hypertension and cirrhosis. Cutaneous changes, such as spider angiomata and palmar erythema (Figure 159-1) and complications of end-stage liver disease, such as ascites and hepatic encephalopathy, are also strongly associated with underlying cirrhosis. While these physical findings have a high specificity for cirrhosis, their sensitivity is low.
Figure 159-1
(A) Spider angiomata. (Reproduced, with permission, from Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York: McGraw-Hill; 2008. Fig. 151-9.) (B) Palmar erythema (Reproduced, with permission, from Wolff K, Goldsmith LA, Katz SI, et al. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York: McGraw-Hill; 2008. Fig. 151-8.)
Laboratory tests that assess hepatic synthetic capacity such as prothrombin time (PT) or international normalized ratio (INR) and albumin concentration may be helpful in making the diagnosis of cirrhosis but can be confounded by variables such as nutritional status and comorbid conditions. Thrombocytopenia in a patient with chronic liver disease is the most sensitive and specific laboratory abnormality for cirrhosis. In patients with chronic hepatitis C infection, a ratio of AST to ALT that is greater than one has a high correlation with cirrhotic changes seen on liver biopsy. Cross-sectional imaging studies of the liver, including computed tomography, ultrasonography, and magnetic resonance imaging, are not sensitive for diagnosing cirrhosis, particularly in its early stages. However, they are reasonably accurate for detecting changes associated with late stages of cirrhosis, such as a small, nodular-appearing liver, splenomegaly, prominent collateral vessels in the portal circulation, and ascites.
|
Patients with cirrhosis may be asymptomatic (compensated) or have symptoms resulting from complications of end-stage liver disease (decompensated). Patients with compensated liver disease have no increase in mortality compared to healthy patients. However, over a 10-year period, approximately 50% of these patients will develop decompensated liver disease. The most common complications of cirrhosis include ascites, variceal bleeding, and encephalopathy. Decompensation dramatically affects survival. The median survival of patients with decompensated cirrhosis is two years.
This chapter focuses on the pathophysiology, diagnosis, and management of four types of cirrhotic decompensation: ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatic encephalopathy. Management of variceal bleeding is discussed in Chapter 155 on Upper Gastrointestinal Bleeding.
Ascites
Ascites is an accumulation of fluid within the peritoneal cavity. Many diseases can lead to the formation of ascitic fluid (Table 159-1). Ascites usually results from either portal hypertension or peritoneal disease. By far the most common cause of ascites is cirrhosis, accounting for 85% of cases. The remaining 15% is caused by peritoneal malignancy, followed by congestive heart failure. Tuberculous peritonitis is a common cause of ascites in endemic regions (developing countries) of the world, but is rarely seen in the U.S.
| Portal hypertension | Cirrhosis |
| |
| Hypoalbuminemia |
|
| Peritoneal disease |
|
| Miscellaneous |
|
Ascites is the most common complication of cirrhosis and the most frequent reason for hospitalization in cirrhotic patients. The development of ascites represents an important transition point in the natural history of cirrhosis, as approximately 15% of patients with cirrhosis die within one year of forming ascites, and 50% die within five years. Many practitioners consider new-onset ascites an indication for liver transplantation evaluation.
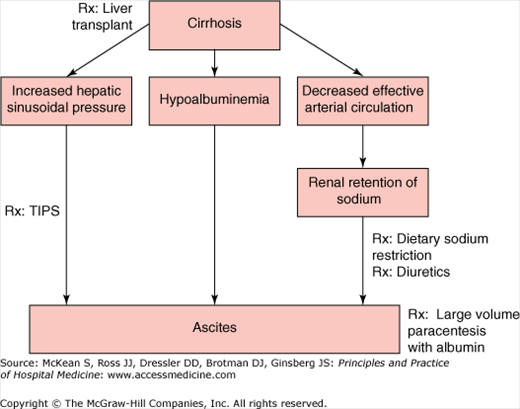
The pathogenesis of ascites in cirrhosis involves two key factors: high pressure in the hepatic sinusoids (portal hypertension) and increased renal retention of sodium (Figure 159-2). Distortion of normal hepatic vascular architecture from formation of regenerating nodules and fibrous tissue results in increased resistance to blood flow and an increase in sinusoidal pressure. Splanchnic arterial vasodilation, mediated by increased production of nitric oxide, increases flow in the vessels entering the liver and exacerbates portal hypertension. Cirrhotic patients with ascites have significantly higher portal pressures than cirrhotic patients without ascites. Ascites develops when the hepatic venous pressure gradient, which estimates intrahepatic vascular resistance, exceeds 12 mm Hg.
Dilation of the splanchnic circulation decreases the “effective” circulating arterial blood volume and activates the sympathetic nervous system. Activation of the renin-angiotensin-aldosterone (RAA) system promotes renal arterial vasoconstriction, increased sodium and water absorption, and plasma volume expansion. Increased plasma volume increases lymph formation through splanchnic capillaries and hepatic sinusoids. Lymph formation is further augmented by hypoalbuminemia, which decreases intravascular oncotic pressure.
Early in cirrhosis, the lymphatic system efficiently returns excess lymph produced in the hepatic and splanchnic compartments to the systemic circulation. Increased lymph resorption is evidenced by dilation of hepatic lymphatics and the thoracic duct in some patients with cirrhosis. However, as cirrhosis progresses, the expanded plasma volume and lymph production eventually exceeds return, and continuous formation of ascites occurs within the peritoneal cavity.
Accurate detection of ascites is difficult based on the physical exam alone. Shifting dullness is the most reliable sign of ascites but is only 77% sensitive and 72% specific for diagnosing ascites. Fluid wave is the most specific sign for ascites (90% specificity), but lacks sensitivity (62%). Dullness in the flanks is only observed when there is at least 1500 ml of ascitic fluid in the abdomen. Because ascites may result from congestive heart failure or constrictive pericarditis, the jugular veins should be carefully examined for evidence of increased right atrial pressure in all patients with ascites. Diagnosing ascites on the physical exam is particularly challenging in patients who are obese or when the volume of ascites is small and usually requires ultrasonographic confirmation. Ultrasonography can also determine a site where a diagnostic paracentesis can be performed safely and provide information about the liver parenchyma and surrounding vasculature.
A diagnostic paracentesis should be performed in the initial evaluation of all patients with new-onset of ascites. It is the most effective and efficient method of determining the etiology of ascites. It should also be performed in all cirrhotic patients with signs of decompensation such as encephalopathy, fever, or renal insufficiency. Infected ascitic fluid can cause or exacerbate all of these complications of cirrhosis. The optimal site for needle insertion is in the left lower quadrant, 3 cm cephalad and 3 cm medial to the anterior superior iliac spine (See Chapter 116, Paracentesis procedure chapter, for more detailed description of Paracentesis procedure). The abdominal wall is thinner and fluid tends to pool at this location. A diagnostic paracentesis is a safe procedure, with serious complications occurring in less than 1/1000 procedures. In a study of more than 1000 paracenteses, no significant bleeding complications occurred, even in the setting of significant thrombocytopenia and prolongation of the prothrombin time. Bleeding complications occur almost exclusively in patients with underlying renal failure, presumably from platelet dysfunction.
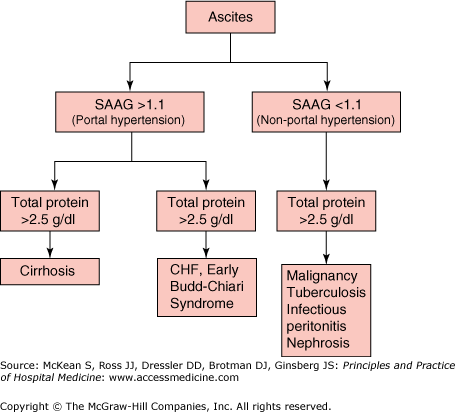
Routine analysis of ascitic fluid (Table 159-2) should include total protein and albumin concentrations and a cell count with differential. Simultaneous serum albumin concentration should be determined in order to calculate the serum-to-ascites albumin gradient (SAAG). The SAAG is the most helpful test in determining the etiology of ascites (Figure 159-3). In patients with cirrhosis and ascites, the SAAG correlates with the degree of sinusoidal hypertension (sinusoidal pressure of 11 mg Hg = SAAG of 1.1 g/dl). If the SAAG is greater than 1.1 g/dl, it is assumed that the sinusoidal pressure is at or exceeds 12 mm Hg and that the ascites is secondary to portal hypertension (presinusoidal, sinusoidal, or postsinusoidal). If the SAAG is less than 1.1 g/dl, the cause of ascites is not related to portal hypertension. Total protein is usually low in cirrhosis (< 1g/dl) and high (> 2.5 g/dl) in most other disease states. Low ascitic protein and albumin in cirrhotic ascites is believed to be secondary to “capillarization” of hepatic sinusoids in cirrhotic livers, where fibrosis occurs within the endothelial lining, obliterating fenestrae and preventing macromolecules such as proteins and complement from entering into the subendothelial space. Spontaneous bacterial peritonitis (SBP) can be diagnosed by a total polymorphonuclear (PMN) cell concentration that exceeds 250/mm3.
|
Based on the results of these initial screening tests, additional tests can be ordered when appropriate. If there is a reasonable index of suspicion for bacterial peritonitis, 10–20 ml of ascitic fluid should be inoculated into blood culture bottles at the bedside (see SBP section). Milky (chylous) ascites should be tested for elevated triglyceride levels, which may suggest lymphatic obstruction, or, rarely, cirrhosis. If the SAAG is low and there are reasons to suspect tuberculosis, it is appropriate to check an adenosine deaminase level and AFB culture. In the setting of peritoneal carcinomatosis, ascitic cytology is positive in more than 95% of cases. Patients with chronic pancreatitis or pancreatic duct leak may form pancreatic ascites when ascitic fluid amylase concentration exceeds serum amylase concentrations. Clinicians should exclude malignancy and tuberculosis as causes of ascites if risk factors and clinical suspicion support these diagnoses, as these diseases are associated with high mortality and require specialized treatments.
Management of ascites (Figure 159-2) in the hospitalized patient depends on the etiology of the ascites. Hospitalized patients with tense ascites due to portal hypertension may benefit symptomatically from large-volume paracentesis. Large volume paracentesis should not be performed in patients with SBP, as it may precipitate renal dysfunction.
To prevent reaccumulation of fluid, the mainstay of therapy for ascites is sodium restriction and diuretic therapy. Mobilization of ascites requires a negative sodium balance, which is determined by dietary intake and renal sodium excretion. The degree of sodium restriction depends on renal sodium retention. Patients who excrete relatively higher amounts of sodium (24-hour urine sodium ≥ 25 mEq) can be placed on a 2g/day sodium restriction. Patient who are sodium avid (24-hour urine sodium < 25 mEq) require 1g/day restriction. Because fluid retention in ascites results from inappropriate renal retention of sodium, free water restriction generally is not necessary unless the patient is hyponatremic, with a serum sodium of less than 125 mEq/L.
Spironolactone and furosemide are the most commonly used diuretics in the management of ascites. They promote natruesis through different mechanisms. Spironolactone acts as a competitive inhibitor of aldosterone in the distal nephron. Furosemide inhibits the Na+-2Cl–-K+ transport system in the ascending limb of the loop of Henle. Simultaneous administration increases the natriuretic effect of both drugs and reduces the incidence of potassium imbalance that can occur when given alone. Diuretics should be initiated with a diuresis goal of approximately 1 liter per day. If the patient does not have signs of peripheral edema, a smaller diuresis goal of 500 cc per day is appropriate, as more rapid removal can lead to plasma volume depletion.
Oral spironolactone is initiated first at 100 mg daily, followed by oral furosemide at 40 mg daily. These medications can be slowly increased at the same ratio if the patient’s renal function tolerates it, to a maximum dose of spironolactone 400 mg daily and furosemide 160 mg daily. A spot urine sodium to potassium concentration ratio that exceeds 1 indicates that a patient has effective aldosterone blockade and should respond to sodium restriction. In contrast to hospitalized patients with ascites due to congestive heart failure, patients with cirrhotic ascites should not be treated with aggressive intravenous diuresis, as this often leads to azotemia.
|
Patients with continued fluid accumulation despite sodium restriction and diuretic therapy have refractory ascites. There are two subtypes of refractory ascites. Diuretic-resistant ascites cannot be mobilized or recurs quickly despite maximal dietary sodium restriction and diuretic doses. Diuretic-intractable ascites is persistent ascites due to limitations in diuretic usage secondary to diuretic-induced complications, such as rise in creatinine or hepatic encephalopathy. Approximately 10% of patients with cirrhotic ascites develop refractory ascites.
Treatment options for these patients include large volume paracentesis (LVP), transjugular intrahepatic portosystemic shunt (TIPS), and liver transplantation. Serial paracenteses, usually 4–6 liters per session, can be effective at symptomatically treating these patients. Albumin replacement during LVP (10 grams per liter removed) remains controversial. Its administration is for prevention of postparacentesis circulatory dysfunction caused by further activation of the sympathetic nervous system and RAA system due to fluid shifts that result in decreased systemic blood volume. Colloid replacement with 25% intravenous albumin appears to be most appropriate when more than 5 liters are removed, and should be initiated during the paracentesis procedure. Placement of TIPS is usually performed by an interventional radiologist. It has been demonstrated to be very effective at decreasing ascites recurrence but it is associated with increased risk of encephalopathy and is poorly tolerated in patients with advanced liver disease.
Nonsteroidal anti-inflammatory drugs (NSAIDS) should be avoided in patients with cirrhosis and ascites because they can lead to sodium retention and renal insufficiency through inhibition of cyclooxygenase and prostaglandin synthesis. This is particularly critical in cirrhotic patients who maintain high renin levels and rely on prostaglandin synthesis to ensure sufficient renal blood flow and adequate GFR.
Spontaneous bacterial peritonitis (SBP) is a bacterial infection of ascitic fluid without an apparent intra-abdominal source of infection, and thought to be due to translocation of bacteria across the bowel wall. Pathogenesis of SBP may involve disruption of intestinal permeability barrier and increased susceptibility of cirrhotic ascites to infection due to low complement concentration. The classic clinical manifestations of SBP include ascites, abdominal pain, fever, and altered mental status. However, patients with SBP may be asymptomatic or present with much more subtle symptoms, such as mild confusion. Because of this and the importance of early treatment to prevent adverse outcomes, all patients hospitalized with ascites should undergo diagnostic paracentesis at the time of admission to evaluate for SBP. Patients should have a repeat diagnostic paracentesis during their hospitalization if they develop clinical symptoms or laboratory abnormalities concerning for infection.
|
The diagnosis of SBP is made through analysis of ascitic fluid (Table 159-3). A neutrophil absolute cell count ≥ 250 cells/mm3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree