Introduction
All patients are entitled to good palliative care, and it is a necessary part of any practitioner’s armamentarium. General clinicians and specialists therefore need a flexible and effective understanding of symptom control that can be applied diversely.
There are three main problem issues in chronic disease:
- The impact of the disease on an individual’s daily living and, conversely, the possibility of improving quality of life by attending to social and practical issues
- The uncertainty of the progression of the disease and often its punctuation with exacerbations of potentially fatal complications
- Ways to modify pathology and manage symptoms.
These three issues translate into:
- Optimisation of the external environment
- Optimisation of the internal environment
- Optimisation of function and control of symptoms.
Key to the optimum way ahead for effective palliation in chronic non-malignant disease has to be effective communication between the relevant specialities. Some of the knowledge we have from working in cancer care can be transferred, though it is naive to think it is just a simple transfer of knowledge. In addition, specialists such as cardiologists, neurologists, renal physicians, and respiratory physicians will always have a key role in the palliation of most of their patients for obvious reasons.
Advanced cardiac disease
At all stages the management of cardiac disease has a substantial palliative component, and, unlike management of cancer, there are few opportunities for cure. This section focuses on palliative care in cardiac failure, as this is the final common pathway in most patients with advanced cardiac disease who do not die suddenly.
The challenge of effectively applying palliative care rests in the unpredictable course in advanced heart failure, the way in which the healthcare system is organised, and the doctor’s understanding of their roles and responsibilities.
Prevalence
Cardiac failure affects 1–2% of the adult population, and the prevalence rises steeply with age (to more than 10% of those aged over 70). It is a disabling and lethal condition that also has a detrimental effect on quality of life. Up to 30% of affected patients require admission to hospital in any year (120 000 admissions annually in the UK). Mortality is higher than in many forms of cancer, with a 60% annual mortality with in patients with grade 4 heart failure and an overall five year mortality of 80% in men.
Clinical aspects
There are several important similarities to and differences from cancer. One key difference, previously suspected and now confirmed, is the more linear and predictable course in cancer. In addition, it is now recognised that anaemia and pain can be regarded more as similarities than differences, and this may have implications for quality of life for patients with advanced heart failure.
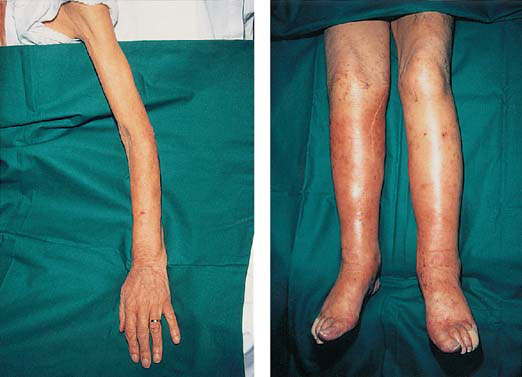
Marked muscle wasting in the arms (left) combined with oedema of the legs (right) in a patient with advanced heart failure
| Cardiac related | Cancer related |
|
|
| Common to both | |
|
|
- Breathlessness, lethargy, cachexia
- Nausea, anorexia, abnormal taste
- Weight loss (loss of muscle mass countered by fluid retention)
- Pain
- Constipation
- Poor mobility
- Insomnia, confusion, depression
- Dizziness, postural hypotension, cough
- Jaundice, susceptibility to infection
- Polypharmacy
- Anaemia
- Abnormal liver function tests
- Fear of the future
- Predicting life expectancy is less easy
- Oedema is a more dominant feature with differing mechanism
- Patients mistakenly perceive it as a more benign condition
Management
Patients will be faced with frequent admissions to hospital. The patient’s preference for management at home must be acknowledged and addressed. The heart failure liaison nurse programme pioneered in Glasgow has been shown to reduce the number of admissions by early detection and management of worsening heart failure and by ensuring that the patient’s home meets all the necessary requirements for optimal home care. The patients have uniformly appreciated the support provided by this system.
Examples of requirement for hospital admission related to the home circumstances and support are:
- Need for intravenous therapy
- Persistent paroxysmal nocturnal breathlessness and orthopnoea
- Refractory oedema and fluid leakage from lower limbs
- Symptomatic postural hypotension
- Development of dysrhythmias.
Dietary advice is important and complex in that the patient may be obese or cachectic. Frequent small meals are preferable, which should be tailored to the patient’s tastes. Tumour necrosis factor and interleukins are implicated in the aetiology of cachexia, and fish oils may reduce their levels. Supplements of fat soluble and water soluble vitamins may also be necessary to counteract the increased urinary loss and reduced absorption. A small amount of alcohol may help as an appetite stimulant and anxiolytic.
Reduction of fluid intake to 1500 ml a day and avoidance of excessively salty foods (but not to the extent of making food tasteless) will help to control oedema. Exercise may reduce breathlessness and improve both quality of life and psychological wellbeing. This must be tailored to each patient’s needs.
Drug treatment
The main emphasis is relief from symptoms: drugs being given to improve prognosis should be reviewed.
Opioids, combined with antiemetic drugs if necessary, are useful for control of nocturnal breathlessness. Awareness of toxicity because of associated respiratory and renal insufficiency is paramount. The role of alternative opioids such as oxycodone has not been established for the easing of dyspnoea. In clinical practice, alternative opioids may be tried if side effects limit the use of morphine. Anxiolytics also have an important role, and achieving the correct balance requires individual tailoring of therapy.
Diuretics also have a key role—orally, intravenously, or in combination depending on the severity of fluid retention. However, awareness of the clinical (fatigue, nausea, and lightheadedness from postural hypotension) and biochemical features of overdiuresis is essential.
Digoxin can relieve symptoms in patients with advanced heart failure, but it is vital that symptoms of toxicity are avoided.
Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blocking agents are beneficial, and the dose should be titrated to ensure maximum benefit without adverse effects. As many patients are volume depleted and hypotensive, small supervised test doses should be given—such as 6.25 mg of captopril or 2.5 mg of ramapril after 12–24 hours without diuretics or equivalent doses of angiotensin receptor blocking agents (definite indication for this group is cough secondary to ACE inhibitors). In patients unable to take ACE inhibitors and angiotensin receptor blocking agents, other vasodilators (such as hydralazine) might be considered, although in this situation they are of marginal value.
- Enlist help of heart failure liaison service if available
- Assess appropriateness of the home—such as comfortable bed or recliner chair, easy access to toilet, family support
- Establish need for oxygen therapy—balance benefits and risks
- Monitor fluid status and appropriateness of diuretic treatment
- Consider normal release opioid at night (for example, oral morphine 5 mg) to ease dyspnoea but use with caution and appropriate adjustment of dose in patients with associated renal or respiratory disease
- For night sedation consider temazepam 10–20 mg, or thioridazine 10 mg or haloperidol 0.5 mg in elderly people
- Assess need for dietary advice, particularly to ensure adequate energy intake
- Ensure optimum treatment of heart failure with emphasis on symptomatic rather than prognostic benefit
- Regularly consider need for hospital admission
Breathlessness
- Oxygen
- Opioids—regular, normal release oral morphine 5 mg, or intravenous diamorphine 2.5 mg if patient is acutely distressed
- Non-drug measures such as fan, positioning, explanation, reassurance
- Diuretics, digoxin
- ACE inhibitors, angiotensin receptor blockers, and other vasodilators
- Cycle of breathlessness and panic may require an anxiolytic
- Physiotherapy
- Assess diet and energy intake
- Reassess drug therapy
- Check for postural hypotension
- Check for drug induced hypotension
- Exclude arrhythmia as a cause
- Analgesics—avoid NSAIDs, consider opioids as above
- Reassess anti-anginal regimen
- Non-drug measures —relaxation, TENS, hot packs, dorsal column stimulator, device therapy
- Check drug treatments
- Check liver function
- Frequent small meals and appetite stimulants such as alcohol
- Consider metoclopramide
- Early detection is important
- Loop diuretics—frusemide remains first choice
- Spironolactone 25 mg if tolerated. Increasing doses may help with control of oedema but watch for hyperkalaemia and painful breasts
- Restrict fluid intake to 1500–2000 ml a day
- Mild salt restriction if tolerated
- Bed rest in early stages; when patient is out of bed, raise lower limbs in a recliner chair
- Aim for weight loss of 0.5–1 kg a day
- Additional diuretic treatments may be needed, such as bendrofluazide 5 mg or metolazone 2.5 mg/day
- Monitor electrolytes
Sublingual glyceryl trinitrate may be helpful during episodes of breathlessness. Influenza and pneumococcal vaccination are worth considering despite the advanced nature of the disease.
Counselling and psychological support
Unlike for those with cancer, there is no highly developed support network for patients with end stage cardiac disease. Counselling is certainly challenging in this setting because of the high incidence of sudden death (up to 50%), as is the misconception of patients, who often underestimate the seriousness of the situation. Application of many of the principles of palliative care is needed to optimise this aspect of management.
End stage renal disease
Definitions, incidence, and prevalence
End stage renal disease or failure (ESRF) occurs when the glomerular filtration rate is insufficient to maintain health, usually when the rate is <10 ml/min. Renal replacement therapy (RRT), dialysis, or transplantation has transformed the lives of patients with ESRF, though the disease remains incurable with 10–20% of affected patients dying each year. In the past 20 years a fivefold increase in the number of patients accepted on to RRT programmes has led to a prevalence of 530 patients per million population. The median age of patients undergoing dialysis has increased from 45 to ?65 in a similar time, and diabetes, once present in just 2% of patients having dialysis, is now the most common cause of ESRF in RRT programmes. This means considerable comorbidity for many patients.
Prognosis and causes of death
Age and diabetes are the key factors determining prognosis. The overall one year survival in patients with ESRF on dialysis is 84%, but the five year survival of a young person who does not have diabetes is 74% while that of someone aged >65 with diabetes is 21%. The most common cause of death is cardiovascular disease. A considerable number of patients choose to stop dialysis, and a further group opts for initial conservative management (without dialysis). Patients who choose to stop dialysis have obvious and urgent needs for terminal care; the average time to death is 10 days. A planned multidisciplinary palliative care pathway, available in some areas, will help patients who opt for conservative management, who have a less well defined time course with an average prognosis of seven months.
Management of pain and other symptoms
At least 50% of patients undergoing dialysis experience pain, which is severe for nearly half of them. Pain is often intermittent but occurs over many years and the diverse causes lead to a high incidence of neuropathic pain. Numerous factors impede good pain control. A similar approach to that used to manage cancer pain can be taken with the WHO analgesic ladder, including adjuvants where indicated. Careful monitoring for toxicity is essential because of the retention of drugs or their metabolites in patients with renal failure. The active morphine metabolite, morphine 6 glucuronide, is retained in patients with ESRF and when morphine is taken for chronic pain its retention can lead to toxicity, including cognitive impairment and myoclonus. Alternative strong opioids—such as oral hydromorphone and subcutaneous fentanyl or alfentanil and transdermal buprenorphine—are being explored. Clearance of fentanyl may be altered in patients with ESRF, though it does not have known active metabolites. Other symptoms are also common, occur over many years, and can be difficult to manage as the evidence is scarce or the remedies toxic.
- Adaptation of the role of heart failure liaison nurses to include palliative care
- Combined care from both palliative care specialists and cardiologists
- Improved understanding of mechanisms and treatment of nausea and cachexia
- Improved understanding of the role of opioids and anxiolytic agents
- Improved recognition of the need for psychological support and counselling
|
|
| Causes of pain in renal failure | Pain related to dialysis |
|
|
| Disease consequent on renal failure | Primary renal disease |
|
|
|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








