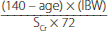Key Clinical Questions
How are the stages of chronic kidney disease defined?
What are the different dialysis modalities, and how do patients choose one over another?
What is the difference between an arteriovenous fistula and an arteriovenous graft? What are their associated complications?
When is it appropriate to initiate dialysis?
How are hemodialysis and peritoneal dialysis performed? What are their common complications?
What complications are common in hospitalized patients with chronic kidney disease?
Introduction
Over 26 million Americans, or approximately one in nine adults, have chronic kidney disease (CKD). These patients require special attention from hospitalists because of a high incidence of acute kidney injury (AKI) and other complications during hospitalization. These patients are at risk of AKI not only from known nephrotoxins, such as contrast agents and nonsteroidal anti-inflammatory drugs (NSAIDs), but also from other commonly prescribed agents. Other major issues in the hospital care of these patients include preservation of venous access, electrolyte and acid-base correction, and anemia management. As many of these patients advance to end-stage renal disease (ESRD) requiring dialysis, hospitalists should have a basic understanding of the principles of renal replacement therapy (RRT).
Chronic Kidney Disease
The National Kidney Foundation has defined a staging system for CKD (Table 248-1) based on glomerular filtration rate (GFR) as the best marker of kidney function. CKD is defined as an absolute GFR less than 60 cc/min, or structural or functional kidney abnormalities eg, hematuria, proteinuria, abnormal imaging) with a preserved GFR (> 90cc/min). Serum creatinine concentration (sCr) is a poor marker of kidney function, as normal values vary with age, gender, and muscle mass. It is especially inadequate in the setting of AKI. In the early stages of serious kidney injury, the sCr may be falsely reassuring, as it may only rise well after the initial insult. GFR is a better marker of kidney function and is used as the basis for the staging system.
| Stage | Description | GFR (ml/min/1.73 m2) |
|---|---|---|
| 0 | At increased risk | > 90 |
| 1 | Kidney damage with normal or increased GFR | ≥ 90 |
| 2 | Kidney damage with mild decrease in GFR | 60–89 |
| 3 | Moderate | 30–59 |
| 4 | Severe decrease in GFR | 15–29 |
| 5 | Kidney failure | < 15 or dialysis |
As GFR is not readily measured, mathematical formulae are used to estimate it. Both the Cockcroft-Gault and the Modification of Diet in Renal Disease (MDRD) formulas are commonly used, with many laboratory reports listing estimated GFR (eGFR) (Table 248-2). The Cockcroft-Gault formula is simple, but often overestimates kidney function. The MDRD is a more arduous calculation, but is more accurate in patients with advanced CKD. However, it underestimates GFR in patients with near-normal sCr. These formulas are only useful when sCr is in a steady state. When the sCr is rapidly changing, as in acute kidney injury, they are completely inaccurate and should not be used. In that case, a 24-hour urine collection for creatinine clearance (CrCl) is a better indicator of kidney function. A 24-hour urine collection is recommended whenever renally excreted drugs possessing significant toxicity need to be administered.
Despite optimal medical care, patients with advanced CKD often progress to ESRD. When feasible, ESRD is best treated with transplantation. When donors are available, this is done preemptively, and dialysis is avoided. If dialysis seems unavoidable, preparation and discussion should start at least one year in advance, involving the patient, primary care physician, family, nephrologist, and other caregivers. However, some patients require acute dialysis in the hospital, with quick education by hospitalists and nephrology teams.
Preparation of Patients for Dialysis
Patients with advanced CKD need instruction about the importance of dietary adherence. Diets for stage 5 CKD are restricted in sodium, potassium, and phosphorus. In the past, CKD patients were told to dramatically limit protein intake, as it was thought that this would slow progression of proteinuric kidney disease. Most experts now agree that a modest protein restriction (0.7 g/kg/day) is likely safe, but more restricted diets pose too great a risk of malnutrition. Salt restriction in patients with CKD cannot be overemphasized. Potassium restriction is also important in advanced disease.
Modalities of dialysis that hospitalists should be familiar with include peritoneal dialysis (PD), traditional in-center hemodialysis (HD), home HD, and nocturnal HD (Table 248-3). These modalities are discussed in detail in the proceeding section. The choice should be based on dialysis access options, patient lifestyle, and patient support network.
| Modality | Description |
|---|---|
| In-center hemodialysis | Patient goes to a dialysis clinic for treatment, typically three times a week |
| Home hemodialysis | Patient or partner needles the patient’s access, and runs his or her treatments on a compact hemodialysis machine, either daily or a scheduled number of treatments per week |
| Nocturnal hemodialysis | Patient goes to a dialysis clinic at night, and undergoes longer hemodialysis treatments by skilled nurses overnight during sleep |
| Continuous ambulatory peritoneal dialysis | Patient does 4 to 6 manual exchanges of dialysate into the abdomen over the course of a 24-hour period |
| Automated peritoneal dialysis | Peritoneal dialysis is done via an automated machine that cycles fluid in and out of the abdomen over a set period of time (usually 8 to 10 hours), often during sleep |
When the need for HD is urgent, dual-lumen dialysis catheters can be placed at the bedside or radiology suite by nephrologists, surgeons, or interventional radiologists. These may be used immediately after confirmation of placement by radiography. The internal jugular and femoral veins are preferred sites. Catheters should not be placed in the subclavian vein, because central venous stenosis may develop, precluding the later use of that upper extremity for surgically created fistulae and grafts. Catheters may be untunneled, or tunneled with subcutaneous cuffs. Tunneling the catheter subcutaneously prior to vein entry decreases the likelihood of skin bacteria reaching the bloodstream and causing infection, and allows these catheters to remain in place for longer periods of time. Catheters are not preferred for long-term vascular access. They have high rates of malfunction and infection (approximately 2 to 4 episodes per 1000 patient days) and are associated with increased patient mortality compared with fistulae or grafts.
Ideally, access for HD is established well in advance of the need to start dialysis, as an arteriovenous fistula (AVF) may need months to mature before it is viable. Patients with CKD should be referred to an experienced access surgeon when their estimated GFR is 20 ml/min/1.73 m2, or when they are expected to require dialysis in the next three to six months.
Long-term vascular access for HD requires identification and preservation of suitable veins over time. Fistula require veins greater than 0.3 mm in diameter on ultrasound mapping. The importance of vein preservation cannot be overemphasized. Many CKD patients will require HD access and should have their veins protected, particularly while hospitalized. To this end, one upper extremity (usually the nondominant arm) should be spared from blood draws, sphygmomanometers, and intravenous catheters (including peripherally inserted central catheter [PICC] lines). Scarring of veins occurs rapidly, often making future access creation impossible.
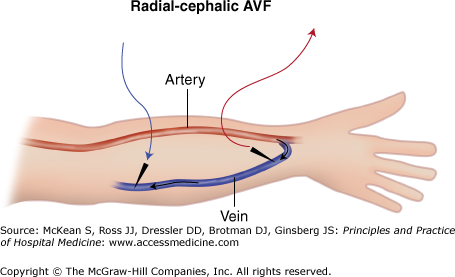
Arteriovenous fistulae are preferred over synthetic arteriovenous grafts because of their longer lifespan and lower infection rate. An AVF is made by surgically anastamosing a native artery to a native vein, usually in the forearm or upper arm (Figure 248-1). Over time, the vein will “arterialize,” so it can withstand thrice-weekly cannulation. Fistula maturation usually requires 6 to 12 weeks. Surgical or percutaneous interventions may be required when maturation is delayed. A successful fistula can last over a decade with low thrombosis and infection rates.
|
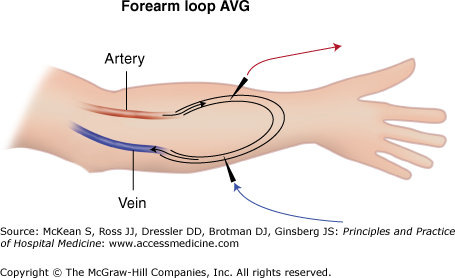
If a suitable vein for creation of an AVF is unavailable, an arteriovenous graft (AVG) made of polytetrafluoroethylene (PTFE) or other synthetic material may be interposed between native vessels as an alternative (Figure 248-2). An AVG can be used for HD two to four weeks after placement. Unfortunately, synthetic PTFE grafts are not as durable as an AVF. On average, grafts thrombose more often and have lower patency rates. Additionally, because these grafts are made of synthetic material, infection usually requires surgical excision and abandoning the access.
Initiation of Dialysis
In some patients, CKD slowly progresses and dialysis can be initiated in a predictable fashion. For others, AKI necessitates urgent initiation of treatment.
There are several life-threatening scenarios that warrant urgent nephrology consultation and initiation of dialysis, with immediate placement of a double-lumen dialysis catheter for access (Table 248-4). These catheters are most often untunneled. In addition, these temporary dialysis catheters are preferred in patients with bacteremias or other endovascular infections. If there are no infectious issues, a tunneled dual-lumen catheter can be placed for dialysis, with lower long-term infection risks.
|
Dialysis in progressive CKD is undertaken to avoid complications of advanced uremia. Current guidelines (and Medicare reimbursement) recommend the initiation of dialysis when creatinine clearance drops below 10 ml/min. Diabetic patients seem more susceptible to the side effects of uremia, and may require dialysis when the creatinine clearance falls below 15 ml/min. In patients with CKD, physiologic derangements begin well before the need for dialysis. Spontaneous decreases in protein intake, anemia, and altered calcium, phosphorus and PTH homeostasis may begin when creatinine clearance is as high as 30 to 40 ml/min. Early nephrology referral helps manage these early complications of CKD and allows for a smooth transition to dialysis. Patients who have been actively managed by a nephrologist well prior to dialysis have better outcomes than patients seen later in the disease process.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree