KEY POINTS
Between 5% and 10% of patients admitted to adult ICUs become chronically critically ill. The burden of chronic critical illness is anticipated to increase dramatically in the next decade as the population ages and more patients survive the acute phase of critical illness.
Advanced age and multiple organ failure due to severe sepsis and multiple trauma are the most significant risk factors for chronic critical illness, especially when complicated by comorbidities and nosocomial complications.
Chronically critically ill patients have distinct physiology compared with more acutely ill patients, including suppressed levels of anterior pituitary hormones, severe depletion of protein stores with muscle wasting, and relative immune compromise.
Important principles of patient management include prevention of infection, protein repletion, limitation of sedating medications, aggressive physical therapy, and careful attention to treating pain and depression.
Liberation from mechanical ventilation is usually achieved with work-rest cycles that are guided by frequent assessments of readiness for weaning and careful monitoring to avoid fatigue. Weaning protocols that include daily periods of unassisted breathing are more efficient than protocols that are based on gradual decreases in pressure support ventilation.
One-year survival for chronically critically ill patients is between 40% and 50% in most cohorts.
Chronically critically ill patients experience a median of four transfers of care after acute hospital discharge, and 74% of days alive during the subsequent year are spent in institutionalized care or receiving professional care at home. After 1 year, only 10% of patients are alive and functionally independent at home.
Costs of care for chronically critically ill patients are extreme during hospitalization and after discharge. Cost savings can be achieved by managing hemodynamically stable patients in dedicated wards or facilities outside of the acute ICU setting with lower nurse-to-patient ratios.
There is often significant discordance in understanding of long-term outcomes between surrogate decision makers and clinicians. The ProVent Score, a validated clinical prediction rule for long-term mortality in chronically critically ill patients, can inform discussions of prognosis in shared decision making.
INTRODUCTION
Advances in medical management and technology have greatly enhanced patients’ ability to survive critical illness and injury. For most critically ill patients, the clinical course is typified by liberation from organ support systems such as vasoactive drugs and mechanical ventilation after reversal of the acute process, followed by a short period of observation before transfer from the ICU to a medical/surgical ward or an intermediate care unit. For a significant number of patients, however, this timely transition to a more stable condition does not occur, and they remain dependent on life-support systems or other ICU services for prolonged periods. These patients often are referred to as the chronically critically ill (CCI). As larger proportions of aging patients are surviving episodes of severe sepsis, the acute respiratory distress syndrome (ARDS), multiple trauma, or acute on chronic respiratory failure, CCI patients are becoming a significant component of the practice of critical care medicine.
CCI patients have a phenotype that is recognizable to critical care clinicians of any discipline (Table 14-1).1 Patients are usually extremely weak and dependent on mechanical ventilation. Their physical appearance is altered by muscle atrophy and generalized edema, and a tracheostomy has been placed or is being contemplated. Sixty-three percent are delirious or comatose,2 and those that are alert report a wide range of symptoms such as pain, dyspnea, thirst, or anxiety, mostly at severe levels.3 They are often cycling through recurring infections, multiple antibiotics, and are being colonized by multidrug resistant organisms. Their families are distressed, frustrated, and exhausted. Finally, their physicians and nurses are equally frustrated and often are challenged to maintain enthusiasm for their care.
Phenotype of Chronic Critical Illness
Prolonged ventilatory failure and ventilator dependence
Hemodynamic instability
Malnutrition
Muscle atrophy Skin breakdown Delirium or coma Anxiety and depression |
Despite having a recognizable phenotype, a common definition for CCI is more elusive. For the purposes of epidemiologic studies or clinical trials, patients are identified by a certain number of days of mechanical ventilation or ICU care, by presence of a tracheostomy for prolonged ventilation, or by transfer to a ventilator rehabilitation unit.4 The actual number of days of ventilation or ICU stay that is considered to meet a threshold of prolonged has varied from 2 to 29 days depending on an investigator’s intuitive sense of what is exceptional or by restrictions of administrative databases.5-7 In 2005, a consensus conference representing physicians, respiratory therapists, nurses, and long-term care hospitals recommended a standard definition for prolonged mechanical ventilation (PMV) of greater than or equal to 21 consecutive days of mechanical ventilation for ≥6 hours per day.8 However, more recent clinical trials involving PMV patients are enrolling patients after 10 or 14 days of mechanical ventilation in order to intervene earlier along the continuum from acute to chronic critical illness.1 Placement of a tracheostomy for prolonged ventilation is considered by many to be a good definition of CCI because clinicians have determined that the patient is unlikely to die or be liberated from mechanical ventilation in a short period.9 However, there is wide variation between centers as to how early and even if to place a tracheostomy in specific conditions.10 When using administrative data such as Medicare datasets that do not include ventilator days, the most reliable approach is to identify patients who have an ICU length of stay of ≥21 days and have been assigned DRG 541 or 542 (tracheostomy for a condition other than head, neck or face disease) or ICD-9 code 96.72 (mechanical ventilation >96 hours).7
INCIDENCE
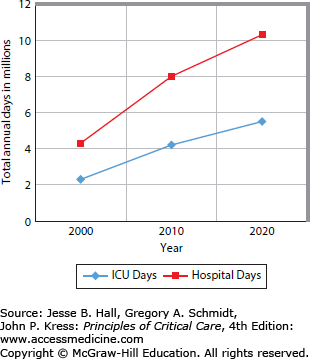
Depending on the definition, between 5% and 10% of patients admitted to adult ICUs become chronically critically ill.11,12 Patients with DRG 541 or 542 increased from 86,911 in 2000 to 116,491 in 2010, an increase of 34%. Total hospitalizations only increased by 7% during that period (AHRQ National Inpatient Sample, 2010. http://hcupnet.ahrq.gov) These CCI patients represent 0.25% of the 35 million annual hospital discharges in the United States. Although this is a small fraction of all hospital admissions, CCI patients have a substantial impact on hospital resources owing to prolonged stays and high-intensity care. Importantly, 52% of CCI patients are over age 65. This reflects an overall higher incidence of acute respiratory failure in elderly patients.13 As the baby boom generation approaches this age group in the next 10 years, the number of patients at risk for CCI is expected to more than double, demanding a significant increase in ICU and hospital bed days (Fig. 14-1).14
FIGURE 14-1.
Projected increases in ICU and hospital bed days for patients requiring at least 96 hours of mechanical ventilation (prolonged acute mechanical ventilation [PAMV]). (Adapted with permission from Zilberberg MD, Shorr AF. Prolonged acute mechanical ventilation and hospital bed utilization in 2020 in the United States: implications for budgets, plant and personnel planning. BMC Health Serv Res. November 25, 2008;8:242.)
RISK FACTORS
Patients who are susceptible to chronic critical illness are as heterogeneous as the general ICU population. The most significant risk factor for CCI is multiorgan failure including shock or ARDS at admission (Table 14-2).15 Severe sepsis and multiple trauma are common etiologies as are severe neurologic injuries such as stroke or traumatic brain injury.16 While PMV is a hallmark of CCI, and patients with end-stage lung disease or neuromuscular disorders are certainly susceptible, such patients with single organ failure represent a small proportion of the CCI population. Patients with postoperative complications from cardiac or abdominal surgery are at risk, and trauma patients are common as well. Critically ill patients admitted to the ICU with significant comorbidities are at higher risk, especially those with underlying heart disease, chronic obstructive pulmonary disease (COPD), and kidney disease. For surgical patients, preoperative instability, COPD, prolonged operation, and in the case of cardiac surgery patients, increased bypass time are important risk factors for PMV.17 Development of nosocomial pneumonia, aspiration events, and failed extubations are additional proven risk factors for PMV.18 A predictive model quantifies the risk of prolonged (greater than 7 days) mechanical ventilation by including the primary disease, acute physiology by APACHE III score, age, presence of COPD, prior functional limitations, and length-of-hospital stay prior to ICU admission.19 The acute physiology score and primary reason for ICU admission accounted for 0.66 of the explanatory power for the model. Of the variables in the acute physiology score, pH, PaCO2, PaO2/FiO2ratio, albumin level, and respiratory rate were significant predictors. Further development of clinically useful prediction models for PMV would be of great benefit for resource planning in the ICU.
Risk Factors for Chronic Critical Illness
|
Perhaps one of the most important risk factors for chronic critical illness is ICU-acquired weakness associated with critical illness polyneuropathy (CIP), critical illness myopathy, and immobility. CIP is evident in up to 47% of patients who are ventilated for greater than 7 days20 and in 95% of patients who are ventilated for more than 28 days.21 The presence of the systemic inflammatory response syndrome (SIRS) and hyperglycemia are the greatest risk factors.22 The use of aminoglycosides, neuromuscular blockers, and steroids may also contribute to the development of CIP although studies are conflicting. Abnormalities on neurophysiologic testing persist for up to 5 years.21 There is no specific therapy for this condition other than aggressive rehabilitation. In most cases, recovery is very slow. Diaphragm paralysis from phrenic nerve injury is another neuromuscular condition that contributes to PMV. It is difficult to diagnose, but it should be suspected in any patient who has had cardiothoracic or neck surgery and has difficulty with spontaneous breathing, especially while in the supine position. An elevated hemidiaphragm on chest radiograph is suggestive, but it is often not present. Real-time ultrasound during spontaneous breathing is a simple and accurate means to establish the diagnosis.21b
PATHOPHYSIOLOGY OF CHRONIC CRITICAL ILLNESS: THE NEUROENDOCRINE MODEL
Despite the varied definitions and nonspecific clinical findings that have been used to describe CCI patients, they appear to be a physiologically distinct subset of the overall ICU population. This has been best demonstrated by the work of Grete Van den Berghe and others who have examined neuroendocrine responses to critical illness. During the acute phase of critical illness, adrenocorticotropic hormone (ACTH), cortisol, and prolactin levels are elevated, whereas thyrotropic and gonadotropic hormone levels are reduced.23 During the chronic phase of critical illness, hormonal responses are significantly different (Table 14-3). ACTH and other anterior pituitary hormone levels decrease, but hypercortisolism persists, suggesting an alternative pathway for cortisol release.24 CCI patients lose thyroid-stimulating hormone (TSH) pulse amplitude, which results in typically low or low-normal TSH levels and low thyroxine (T4) and triiodothyronine (T3) concentrations compared to acutely stressed patients. This may be related to reduced expression of the thyrotropin-releasing hormone (TRH) gene in the hypothalamic paraventricular nuclei.25
The somatotropic axis also demonstrates important differences between acute and chronic critical illness. For patients who are in the acute phase of critical illness, the pituitary gland actively secretes growth hormone (GH) into the circulation in a pulsatile fashion that is regulated by hypothalamic growth hormone–releasing hormone (GHRH). GH levels and GH pulse frequency are increased compared with normal function. In contrast, for patients who have received mechanical ventilation for greater than 21 days, the pattern of GH secretion is less regular, and the amount that is released in pulses is greatly reduced.23 Nocturnal secretion of GH is reduced relative to the acute stressed condition.
The hormonal changes that occur in acute illness may be positive adaptations that help divert energy away from anabolism and toward maintenance of vital tissues and immune function, for example. However, the hormonal responses to chronic critical illness may be maladaptive. CCI patients suffer from significant protein deficiencies owing to ongoing degradation and suppressed production. This hypercatabolic state likely contributes to the severe and prolonged muscle weakness that is characteristic of these patients. While protein is lost despite feeding, reesterification of free fatty acids allows fat stores to build up.23 Hyperglycemia, insulin resistance, and hypertriglyceridemia are common. Prolonged hypercortisolism and low levels of GH and thyroid hormone may contribute significantly to these processes. In addition to prolonged wasting, immune function is also likely to be affected as well. As a clinical correlate, prolonged weakness associated with ventilator dependence and recurrent infectious complications are hallmarks of the CCI condition.
MANAGEMENT OF THE CCI PATIENT
CCI patients are at very high risk for nosocomial infection. Perhaps their greatest risk factor is disruption of multiple infection barriers. Most patients have tracheostomies or endotracheal tubes that promote aspiration, inhibit cough, and greatly increase their risk of airway colonization with nosocomial organisms. Central venous catheters, including peripherally inserted central catheters (PICC lines), are common and remain in place for long periods, significantly increasing the risk of bloodstream infections. The presence of bladder catheters promotes urinary tract infections, and nasogastric tubes increase the risk of sinusitis. Weeks of immobility and edema predispose patients to skin breakdown, which provides another infection source.
The underlying comorbidities that make patients susceptible to chronic critical illness also predispose to infections. COPD is often accompanied by bacterial colonization of lower airways and compromised airway clearance. Neurologic impairment increases aspiration risk and weakens cough response. Diabetes mellitus, renal failure, congestive heart failure, and hepatic dysfunction are all associated with compromised immune function and are important risk factors for pneumonia. Diabetes mellitus and hepatic dysfunction also increase the risk for fungemia. Immune function is further impaired by nutritional deficiencies, protein depletion, and ongoing catabolic processes. Recent data indicate that “immune exhaustion” following severe sepsis can leave patients effectively immune compromised as soon as 3 to 4 days following their acute presentation.26
Because CCI patients spend weeks in ICUs where multidrug-resistant bacteria are common, the incidence of infection or colonization with these organisms is quite high.27 This problem is compounded by multiple rounds of broad-spectrum antibiotics over the course of their hospitalization. This is a particularly important issue for ventilator rehabilitation hospitals, where patients are admitted from numerous different referring hospitals. Nearly every new admission brings unique strains of resistant organisms. Containing the spread of these organisms is a constant challenge.
Infectious complications were documented in a series of 100 patients admitted to a hospital unit dedicated to the care of patients with chronic critical illness.28 All patients were receiving mechanical ventilation through a tracheostomy after at least 2 weeks of critical illness. During hospitalization on this unit, 61% of patients developed evidence of SIRS, and 11% developed septic shock. Line sepsis (11%), primary bacteremia (6%), tracheostomy-associated pneumonia (10%), and Clostridium difficile colitis (10%) were the most common infections. Urosepsis was less common in this series, but the authors were appropriately conservative in the diagnosis of urosepsis. This diagnosis required the presence of SIRS and pyuria or a positive urine culture without any other obvious source of infection.
The management of nosocomial infections in CCI patients begins with prevention. Elimination of all unnecessary compromise of barriers to infection is paramount. Venous catheters should be well maintained and removed as soon as possible. A patient who is hemodynamically stable with a functioning gastrointestinal tract may be able to receive all medications enterally. Continued maintenance of a venous catheter out of habit or ICU policy in a hemodynamically stable patient is inappropriate. When central venous catheters are necessary for long periods, tunneled catheters or chlorhexidine-, silver-, or antimicrobial- impregnated catheters should be considered. More importantly, standardized and checklist-monitored approaches to line placement and maintenance should be adopted.29 Bladder catheters should be removed as soon as possible. Strategies to prevent ventilator-associated pneumonia include semirecumbent positioning, oral decontamination, removal of the nasogastric tube when possible, closed endotracheal suctioning, and scheduled drainage of condensate from ventilator circuits.30 Minimizing sedation is also beneficial.31 As always, effective hand washing is essential. Judicious use of broad-spectrum antibiotics, reduced reliance on proton-pump inhibitors, and effective isolation will decrease the incidence of C difficile colitis and infections with multidrug-resistant organisms. Routine surveillance for these organisms may be beneficial; however, a recent cluster randomized trial of ICUs revealed that universal decontamination using 5 days of twice-daily intranasal mupiricin and daily bathing with chlorhexidine-impregnated cloths was more effective than targeted decontamination or screening and isolation of colonized patients in reducing rates of MRSA clinical isolates and bloodstream infections of any type.32 It is yet to be determined whether similar results would be obtained in a ventilator weaning facility where colonization with resistant organisms is more common.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree