Abdominal cutaneous nerve entrapment syndrome
Abdominal wall hernias and post-herniorrhaphy pain
Surgical scars and neuromas
Abdominal wall or rectus sheath hematoma
Thoracic disc degeneration and thoracic radiculopathy
Herpes Zoster infection and post-herpetic neuralgia
Chest wall pain etiologies, slipping rib, or ribs on pelvis syndrome
Abdominal wall endometriosis
Referred pain from an abdominal or thoracic source
Clinical Presentation and Diagnosis
CAWP is best diagnosed based on patient’s history and a physical examination. An important finding is that the pain is usually well localized with point tenderness on palpation. On the contrary, visceral pain is usually more dispersed and poorly localized [5].
Carnett’s test is the hallmark of the physical examination for diagnosing abdominal wall pain [6]. The patient is placed in the prone position with slightly flexed knees and hips to relax the abdominal wall. The painful area is palpated while in this relaxed position, then the patient is asked to tighten his abdominal muscles by staining or lifting his head and shoulders off the bed. A positive test is demonstrated by increased tenderness as the patient tenses the abdominal wall indicating that the pain arises from the abdominal wall. On the other hand, when pain arises from an intra-abdominal source, the tensed abdominal wall muscles guard the underlying organs, thus reducing the pain.
A working clinical diagnosis of CAWP can be confirmed by a positive response to trigger point injections or nerve blocks. A successful injection after a positive Carnett sign was needed to be one of the most cost-effective procedures in gastroenterology [5]. Limitations to this approach are the high placebo response to injections especially in pain patients [7] and visceral abdominal disease with involvement of the peritoneum may give a false positive Carnett test as well [8].
Others advocated the use of differential epidural block to allow characterization of chronic abdominal pain into visceral and non-visceral pain.
Differential Epidural Block
Differential epidural block is a diagnostic nerve block that was initially described in 1964 for the evaluation of lower-back and lower-extremity pain [12]. Since then, several modifications of the procedure have been implemented using both subarachnoid and epidural approaches.
Differential epidural block involves the placement of a thoracic epidural catheter and the injection of saline (placebo) and different concentrations or incremental doses of local anesthetics. The procedure relies on the variable sensitivity of nerve fibers of various size, myelination, and function to local anesthetics. Sympathetic fibers and visceral afferent nerves are relatively more sensitive to local anesthetic blockade than large sensory or motor fibers (Table 18.2).
Table 18.2
Interpretation of differential epidural block
1. Visceral pain: No pain after surgical anesthesia of the relevant dermatome with persistent pain relief after the dermatomal somatic anesthesia level recedes |
2. Somatosensory: No pain after surgical anesthesia to the relevant dermatome with the return of pain as somatic dermatomal anesthesia level recedes |
3. Central: Persistent pain in spite of surgical anesthesia |
4. Mixed: mixed picture between the above three scenarios. Often encountered secondary to the subjective nature of pain |
5. Placebo responders: prolonged pain relief with saline injection |
Pitfalls of the Differential Epidural Block Test
1.
The interpretation of the differential block is non-standardized.
2.
The interpretation of the differential block is very subjective.
3.
The interaction between local anesthetic and nerve fibers is a dynamic and unpredictable phenomenon that may be influenced by a multitude of factors.
4.
Overlap in the range of nerve fiber sizes makes it unlikely that any fiber type can be reliably isolated by this procedure.
5.
As a result of the above, the interpretation of the test is often mixed (visceral/somatic/central), which defeats the purpose of the study!
6.
The procedure takes between 4 and 8 h and requires continuous monitoring of the patient.
7.
It has the limitations and disadvantages of neuraxial blocks.
Transversus Abdominis Plane Block for Chronic Abdominal Wall Pain
The transversus abdominis plane (TAP) block is very appealing as a valuable test in diagnosing pain stemming from the abdominal wall and thus helps differentiating somato-sensory from visceral origin of pain [13].
The TAP block is a new regional anesthesia technique that provides analgesia to the abdominal wall. First described in 2001, the technique involves the injection of local anesthetic into the plane between the internal oblique and transversus abdominis muscles, the TAP [14, 15]. TAP block targets the entire anterolateral abdominal wall between the costal margin and inguinal ligament [16]. The introduction of ultrasound-guided TAP block allows the successful installation of local anesthetics around the anterior branches of the thoracolumbar ventral rami blocking “somatic sensations” from the anterior abdominal wall. As stated above, the limitations of differential epidural block are numerous and, contrary to TAP block (somatic) and celiac/hypogastric block (visceral), different nerve fibers cannot be reliably isolated. The author has found that transversus abdominus plane (TAP) block is very valuable in diagnosing pain originating from the abdominal wall and differentiating somatosensory from visceral pain [13]. Single injection as well as continuous infusions can be used for treatment of various abdominal wall pain syndromes [17].
Figure 18.1 offers a suggested algorithm with incorporation of TAP block in the diagnosis and management of chronic abdominal pain.
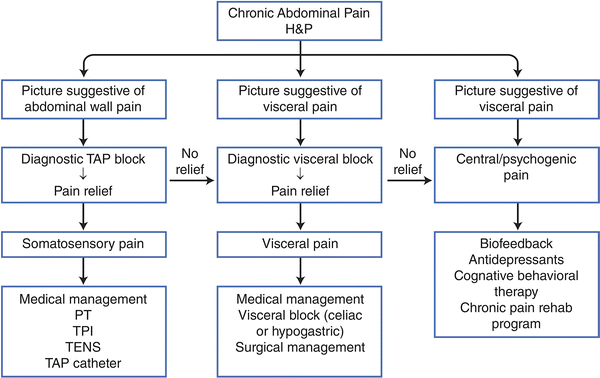

Fig. 18.1
Suggested algorithm for the management of chronic abdominal pain
Anatomy and Innervation of the Anterior Abdominal Wall
The intercostal nerves run a very tortuous course through the abdominal wall muscle. After turning at a 90° angle, the nerve passes from the posterior sheath of the abdominal wall muscle (rectus abdominis) through a fibrous opening and then branches at right angles while passing through its anterior sheath. It has been thought that the underlying problem is nerve compression with resulting ischemia or lack of blood supply, explained by the nerve’s course through the muscle. Applegate termed the condition as “anterior cutaneous nerve entrapment syndrome” and suggested the entrapped nerve may also be pushed by intra- or extra-abdominal pressure or pulled by a scar causing pain in the abdominal wall.
The abdominal wall consists of three muscle layers; the external oblique, the internal oblique, and the transversus abdominis and their associated fascial sheaths. These muscles are innervated via the ipsilateral ventral rami of T7-L1 thoraco-lumbar nerves.
After emerging through the intervertebral foramina, they follow a tortuous course through the abdominal wall muscles. They enter a fascial plane between the transversus abdominis and the internal oblique muscles what is known as the TAP accompanied by blood vessels. This neurovascular plan continues as far as the semilunar line. At the lateral border of the rectus, abdominis muscle, the external oblique and the anterior lamella of the internal oblique aponeuroses pass anterior to the muscle forming the anterior rectus sheath. The aponeuroses from the posterior lamella of the internal oblique muscle and the transversus abdominis muscle pass posterior to the rectus muscle forming the posterior layer of the sheath. At this point, the ventral rami of the thoracic spinal nerves are located between the posterior border of the rectus muscle and the posterior rectus sheath. They run medially within the sheath, through the rectus muscle, then branches at right angles while passing through its anterior sheath [16]. It has been postulated that the nerve’s course through the muscle make it vulnerable to compression and entrapment. Applegate termed the condition ACNES and suggested that the entrapped nerve may also be pulled by a scar or pushed by an intra-abdominal or extra-abdominal pressure causing abdominal wall pain [4].
The Classic Approach for TAP Block
The TAP block was first described by Rafi and McDonell as a blind “double-pop” technique using a blunt needle introduced through the external and internal oblique muscles and fascia at the ilio-lumbar triangle of Petit [14, 15]. This triangle is bounded posteriorly by the latissimus dorsi muscle and anteriorly by the external oblique, with the iliac crest forming the base of the triangle. The introduction of ultrasound allows modification of this technique and the TAP can be accessed anywhere between the iliac crest and costal margin behind the anterior axillary line. A higher subcostal approach may block the upper thoraco-lumbar nerves more effectively than a lower approach immediately above the iliac crest.
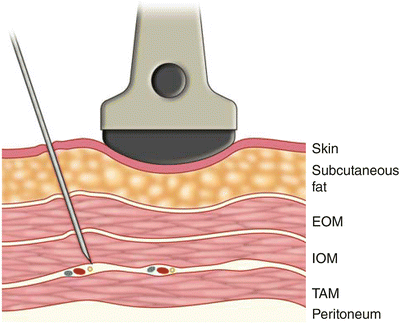
Ultrasound-Guided Technique for TAP Block
The patient is positioned in the lateral decubitus position with the side to be blocked upward. A wedge can be placed underneath the patient in order to stretch the flank on the upper side. A high frequency or lower frequency transducers may be used according to body habitus. Pre-procedural scanning of the anterior abdominal wall along the midaxillary line is recommended to decide the best view of the three muscle layers. Care should be taken that scanning more medially may only show two layers of muscles since the external oblique muscle forms an aponeurosis that joins the rectus sheath. From superficial to deep the following structures are recognized: skin and subcutaneous fat, external oblique, internal oblique, and transversus abdominis muscles with their investing fascia (Figs. 18.2 and 18.3). Deeper to the transversus abdominis and its fascia, there is the pre-peritoneal fat separating it from the peritoneum and the bowels, which are often identified by its peristaltic movements. With ultrasound, the fascial layers appear as hyperechoic layers, and the muscles are identified by their relative hypoechoic structure with multiple striations.