CHAPTER 66
Choosing Intrathecal Medication
INTRODUCTION
• Intraspinal delivery of drugs via implantable drug delivery systems (IDDS) has been available since the early 1980s.
• These devices allow for precise delivery of drugs for treatment of pain and spasticity.
• The delivery is medications in the intraspinal or intrathecal space allows delivery of medications directly to their site of receptor binding in the spinal column at much smaller doses than systemic administration.
• The result is potent analgesic effect with lower systemic side effects.
IDDS comprises of a pump implanted in a subcutaneous pocket usually in the abdomen and attached to a catheter that is tunneled under the skin and inserted in the intrathecal space of the spinal cord at the appropriate level (Figure 66-1). The pump available is programmable or nonprogrammable. The nonprogrammable pump runs at a constant flow rate with dose adjustments made by changes in the concentration of medications. The programmable pump is programmed to deliver the drug or admixture at a controlled rate based on the drug concentration and dose requirements of the patient.
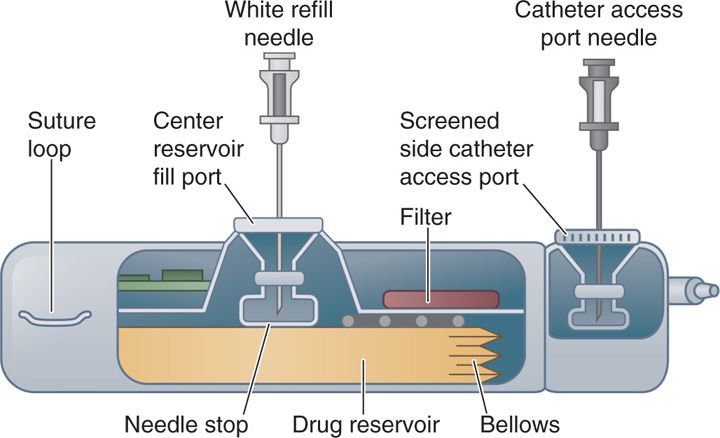
Figure 66-1. Side view of implantable drug delivery device.
Complications related to the pump and catheter system:
• Complications should be addressed prior to choosing and adjusting intrathecal drug medications.1
• The most common operational problems are related to either pump programming or motor stalling.
• Pump programming errors are most likely to occur when drug concentrations are changed.
• Verification of drug concentration, daily dose, volume, and refill date is absolutely necessary.
• A motor stall is typically caused by gear shift wear. The incidence is low (0.5%-2.2%) and can be detected with radiographic examination.2
• New SynchroMed pumps are equipped with electronic logs to detect and report motor stall issues.
• Catheter malfunctions include kinks, holes, or blockages that prevent accurate delivery of medications.1
• Other complications include catheter fibrosis or inflammation and granuloma formation.
Granuloma:
• Development of noninfectious inflammatory mass or granuloma has been reported 0.1% to 5% of patients with implantable pumps.3,4
• Physicians should be suspicious of possible granuloma if patient complains of increased pain that was previously controlled and/or neurological deficits of weakness and sensory loss.
• Granulomas have been reported most frequently with morphine followed by hydromorphone and fentanyl.
Once patient has selected for intrathecal drug therapy, drug selection is the next step. The polyanalgesic consensus conference (PACC) panel has developed recommendations on the rational use of intrathecal analgesics. This PACC panel has reviewed preclinical and clinical research and experience in 2000, 2003, 2007, and 2011.5 The current standard of care of intrathecal therapies reflects on current knowledge from literature and clinical experience. Analysis of published literature is combined with clinical experience of a large panel of scientist and clinicians to form recommendations regarding the use of intrathecal analgesics to treat chronic pain. It is this author’s belief to follow these recommendations as a guide and experience in clinical practice.
PACC 2011 recommendations for prevention and detection of granuloma:
• Prevention
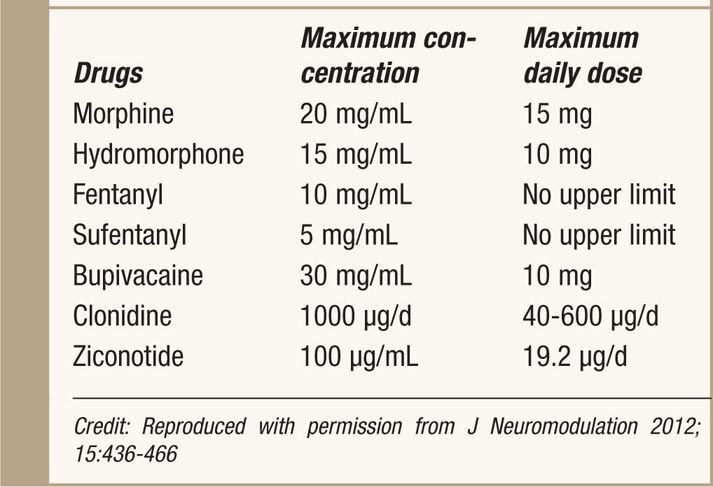
![]() Use lowest effective conc. and dose (Table 66-1)
Use lowest effective conc. and dose (Table 66-1)
TABLE 66-1. 2011 PACC Recommendations for Maximum Daily Doses and Concentrations
![]() Use bolus dosing instead continuous infusion
Use bolus dosing instead continuous infusion
![]() Consider placing catheter tip in lumbar region below conus medullaris
Consider placing catheter tip in lumbar region below conus medullaris
![]() Implement nonopioid adjuvants if concerned about granuloma formation
Implement nonopioid adjuvants if concerned about granuloma formation
![]() Switch IT opioid to Ziconotide if recurrence of granuloma.
Switch IT opioid to Ziconotide if recurrence of granuloma.
• Screening/detection
![]() Detailed history and physical examination every 3 months.
Detailed history and physical examination every 3 months.
![]() Routinely monitor patients for prodromal clinical signs or symptoms.
Routinely monitor patients for prodromal clinical signs or symptoms.
![]() Monitor yearly increase in daily dose.
Monitor yearly increase in daily dose.
![]() Educate other physicians/radiologists about granuloma.
Educate other physicians/radiologists about granuloma.
PACC 2011 panel recommendations:
• The current 2011 PACC panel recommendations have new algorithm tracks for neuropathic and nociceptive pain states is an new step in improving patient care (see Tables 66-2 and 66-3).5
TABLE 66-2. 2011 Polyanalgesic Algorithm for Intrathecal Therapies in Neuropathic Pain
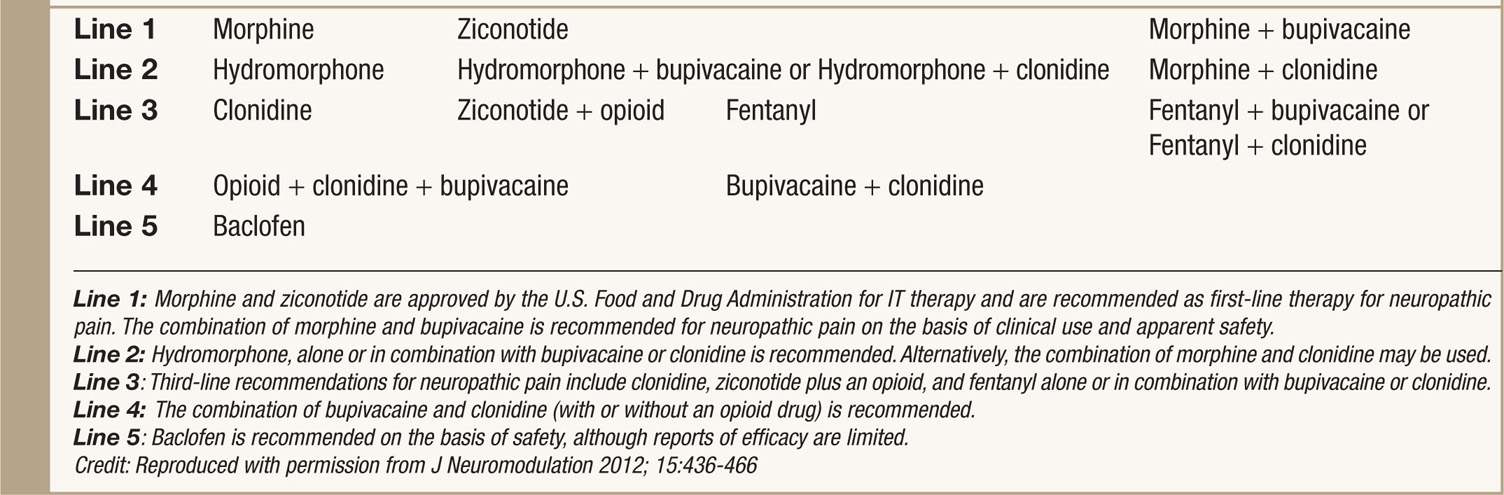
TABLE 66-3. 2011 Polyanalgesic Algorithm for Intrathecal Therapies in Nociceptive Pain
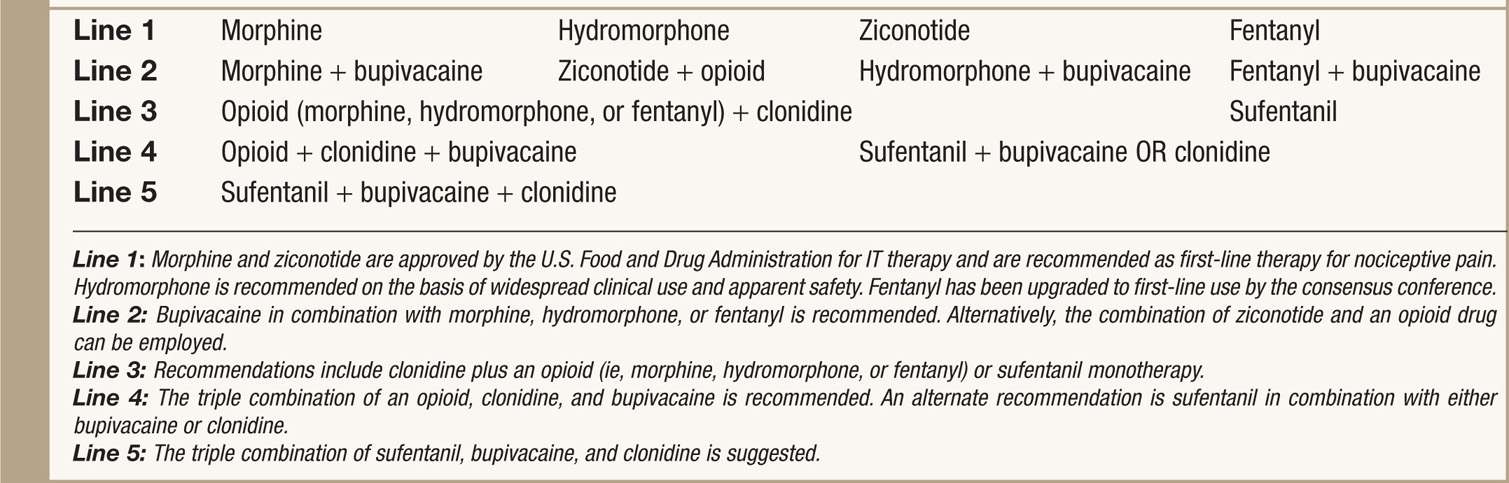
• Prior and current recommendations for drug selection provide clinical practice guidelines for the optimization of IT (intrathecal) therapy with single and multidrug combination in rational and prioritized order.
• In each arm of the algorithm, the medications are arranged in a hierarchy on the basis of efficacy and safety.
• First line medications/combinations are supported by extensive clinical experience and published clinical and preclinical literature.
• Typically, these medications are used to start IT therapies.
• Note that morphine and ziconotide are the two agents that approved by U.S. Food and Drug Administration (FDA) for IT therapies.
• Nonapproved agents are commonly used among pain practitioners who manage IT pump and have been thoroughly discussed for its safety and efficacy.
• Specific recommendations for ziconotide for neuropathic pain and baclofen for pain from spasticity.
• Recommendation made against using certain drugs intrathecally due to toxicity (Table 66-4).
TABLE 66-4. PACC 2011 Recommendations Against Using These Drugs Intrathecally

• Best practices for improved patient care and outcomes with intrathecal infusion are recommended to minimize the morbidity and mortality.
OTHER CONSIDERATIONS
Recommendation Starting Dosages
• Starting dosages recommended by the PACC panel are shown in Table 66-5.5 Appropriate starting dosages may vary on the basis of patient’s baseline oral intake at the time of IT therapy is started.
TABLE 66-5. Recommended Concentration and Doses of Intrathecal Agents by PACC 2011
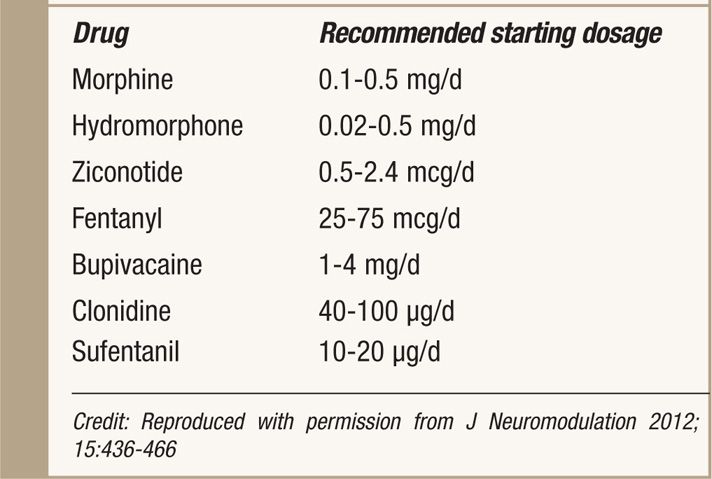
Compounding of Medications
• The U.S. FDA regards traditional pharmacy compounding as the combining or altering ingredients by a pharmacist in response to a licensed practitioner’s prescription so as to treat a patient’s special medical needs.5
• When prescribing a drug for IT administration, special and strict requirements are needed to ensure patient safety and protection of the practice.
• IT drug preparation requires strict sterile and free of contaminants to avoid potential catastrophic effects.
• It is important that the compounding pharmacist has written policies and procedures established and updated to remain compliant with USP 797 standards. This means compounding sterile products under manufacturer’s labeled instructions and with other manipulations that expose the original contents to potential contamination.
• Other considerations in evaluation and selection of a compounding pharmacy for IT drug preparation are the following: aseptic training of personnel, segregated sterile compounding area, environmental monitoring of air quality, calibration of equipment, clean and disinfection program, and quality assurance program.
Drug Admixtures
• The PACC took into account drug stability information available.5
• There is infinite number of drug combinations and it is the author’s view that only drug mixtures be utilized where drug stability information is available.
• If a study suggests that high concentration drug combinations are stable, stability can be assumed for lower-concentration combinations of the same drugs.
• For most pharmaceuticals, there are established and published standards of solubility at room temperature.
• While high drug combinations allow for longer refill interval and delivery of higher daily doses, alteration in pH to develop high drug concentration solutions can result in unstable solutions that can lead to patient and complications with the pump.
• This author has had precipitant cause catheter obstruction and failure of IT drug therapy (see Figure 66-2).6,7

Figure 66-2. Corrosion of pump components due to incompatible drug formulations. (Reprinted with permission from Medtronic.)
• These concentration and pH can be deleterious effect on the pump catheter and tubing.
• This author has seen pump corrosion with high concentration of opioids (see Figure 66-3).8

Figure 66-3. Distal catheter obstruction due to precipitant formation. (Reprinted with permission from Medtronic.)
• A maximum recommendation concentration and starting doses of IT drugs is cited by PACC for the concern of solubility, stability, and granuloma (see Table 66-5).5
• These concentrations are guidelines that can be adjusted for each patient.
• This author may use higher concentrations for cancer patients to accommodate in rapid escalation pain in the end of life.
PACC 2011 Recommendations for Compounding IT Medications
• Avoid preservatives, antioxidants, and solubility enhancers, as they may be neurotoxic.
• Use buffers that are compatible with the delivery system. Acetate buffers are not compatible with the SynchroMed infusion system.
• Use a pH that is physiologically appropriate and is consistent with the drug solubility and delivery system, generally in the range of pH 4 to 8.
• Use solutions that are isotonic with normal CSF (˜300 mOsm/L).
• Prepare the solution in a manner that does not alter the solubility of the constituents to minimize neurotoxicity or incompatibility with pump.
• Verify the chemical and physical stability, and sterility of the preparation in accordance with the USP and ASHP publications.
• Ensure appropriate control of bacterial endotoxins (pyrogens).
Drug Flow Rates and Intermittent Bolusing
Continuous IT delivery of medications is a standard mechanism. No studies exist to support the best rate of delivering medications. Some PACC members theorize that lower flow rates may result in higher concentration of the drug at the catheter tip and may result in higher risk of granuloma, but this has not been proven clinically.5,6
• Constant flow pumps cannot be used to change flow rate or administer boluses. With programmable pumps, intermittent bolusing and patient controlled analgesia (PCA) is now available.
• Bolus doses can be given by programming the pump to give doses at set times and when available, the patient has option of self-administer boluses as needs as a (PCA) or personal therapy manager (PTM).
• Usually boluses are 5% to 20% of daily dose that is administered at a continuous rate. This manner of drug administer is favored given a lot of patient’s pain is intermittent affected by certain activities of daily living and time of day.
• Caution must be used as baseline doses climb with clonidine and bupivacaine.
• To avoid hypotension or motor block, the dose of clonidine should not exceed 20 mcg and the dose of bupivacaine should not exceed 3 mg.
Trialing to Consider Permanent Intrathecal Therapy
The concept of trialing before implantation of an IT drug delivery device is based on the assumption that it will provide information on the efficacy of the therapy. Trialing was thought to be critical prior to implantable and is now debatable. Trialing may be considered in patients with existing implanted pumps for which a change in IT medication is being considered.5
• Trialing may be performed prior to consideration of pump implantation.
• The issue of opioid-induced hyperalgesia (OID) cannot be addressed during a single shot trial or short-term 72-hour drug infusion.
• Many PACC members felt trialing not needed in end-of-life cancer patients if patients have previously tolerated the same drug by another route.
• This author feels that trialing should still be considered for most patients.
• The trial of various medications such as ziconotide will benefit patient beyond potential side effects and toxicities.
• Trialing will allow patient to determine overall benefit in pain relief measured with quality of life and activities scales.
• Realistic expectations can be built during trial for long-term IDDS to reduce patient’s pain and to improve quality of life.
• Trialing can be performed by IT or epidural injection or infusion for most drugs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






