Fig. 8.1
(a) CEUS of normal kidney. In the arterial phase, the cortex shows the most intense enhancement. Note the absence of enhancement in the renal pelvis. (b) CEUS of normal liver in the venous phase. In this phase, the liver appears homogeneously perfused, with the vessels and border clearly defined. (c) CEUS of normal spleen in venous phase. In this phase, the parenchyma appears homogeneous with a persistent enhancement for up to 5–7 min. (d) CEUS of normal pancreas. In the venous phase, pancreas has a darkened appearance (arrows) in contrast to the adjacent liver, but the vessels (asterisk) allow to identify it
In CEUS, the normal parenchyma appears homogeneously hyperechoic with the vessels having the maximum of echogenicity. The enhancement starts 10–15 s after the UCA injection, the time of delay depending on the specific vascular physiology of the investigated organ.
The kidneys show rapid, intense, and transient enhancement due to the absence of glomerular filtration after IV UCA injection. The arterial phase of CEUS starts 10–15 s after intravenous injection and lasts up to about 40 s, when the venous phase becomes prevalent. The venous and late phase lasts from 3 to 6 min. In the arterial phase, the cortex shows the most intense enhancement, whereas in the late phase the whole kidney appears homogeneously perfused.
In the liver, UCAs are firstly visualized in the hepatic artery, followed by those in the portal vein. Hence, the CEUS process is always divided into the arterial phase (<30 s from the injection of UCA), portal phase (31–121 s), and late phase (>120 s). In the portal phase, the liver appears homogeneously perfused, with slightly hyperechoic vessels and anechoic gallbladder. The delayed phase is particularly useful for characterization of focal liver lesions since almost all malignant lesions are hypoechoic in this phase. Also traumatic lesions are well visible in the portal and delayed phase.
Splenic parenchyma starts about 12–15 s after UCA injection. In this phase, we can observe an inhomogeneous enhancement of the spleen, resembling the well-known zebra-striped pattern seen on dynamic CT. The phase can give the false impression of a scattered spleen, confusing the sonographer: we suggest studying first the left kidney and then moving to the spleen in the venous phase. Approximately 50 s after the injection, the venous phase starts, and the splenic parenchyma becomes homogeneous, showing dense persistent enhancement for up to 5–7 min. In this phase, the injured parenchyma is well detectable as a hypoenhanced area.
In the pancreas, uptake of contrast medium during CEUS is very rapid; at approximately 25–40 s, it produces a transient, bright homogeneous enhancement that is due to the high vascularization of the organ. Accumulation in the capillaries is negligible; thus, the washout also occurs rapidly after the arterial phase, giving the pancreas a darkened appearance in contrast to the adjacent liver after 2 min. Consequently, CEUS may be difficult at delineating masses, but it allows an excellent delineation of traumatic lesions.
Tip and Tricks
A double injection of UCA is needed for studying all the organs in all the phases
In the arterial phase, attention must be focused on the vessels in order to highlight vascular injuries and UCA extravasation!
UCAs are not eliminated from the kidney and cannot visualize lesions of the pelvis and ureter
8.2.3 Traumatic Lesions (What We Have to Search for)
Liver
Liver injuries include contusion (subtle and inhomogeneous area without vessel displacement), laceration (clear band-like lesion, linear or branched), and parenchymal or subcapsular hematoma (fluid collection of variable attenuation and echogenicity within liver parenchyma or below the liver capsule).
On CEUS, liver lesions appear as markedly hypoechoic lines or bands and are more evident than on baseline sonographic scans, also showing sharper borders (Fig. 8.2). Injury conspicuity increases progressively while passing from arterial phase scans (20–50 s from injection) to portal-sinusoidal scans (50–240 s), owing to a progressive increase in parenchymal echogenicity. On early-phase images, subtle hyperechogenicity (hypervascularity) can sometimes be noted around the injury, suggesting perilesional hyperemia. In lacerative-contusive areas, CEUS allows optimal depiction of defined lacerations, but in comparison with CT, CEUS less effectively depicts the subtle contusive inhomogeneity. In a series of 87 patients, CEUS was more sensitive than unenhanced sonography in directly showing hepatic lesions (87 % vs. 65 %, 100 % specificity) and correlated better with CT for injury size and capsule involvement.
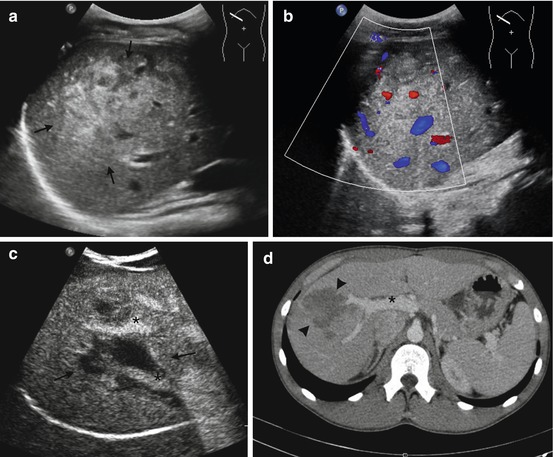

Fig. 8.2
A 19-year-old male admitted to hospital after a motor vehicle accident. (a) Sagittal oblique sonogram shows a large nonhomogeneous hyperechoic area in the right lobe of the liver (arrows). (b) Color Doppler US shows the absence of vascularization. (c) CEUS scan in the same position illustrates a large parenchymal laceration (arrows). The hepatic vessels (asterisks) are in the area of the lesion, but there is no blushing. (d) MDCT confirms the lesion (arrowheads) and the absence of bleeding
Hepatic lesions lack or have very little enhancement, appearing as hypoechoic areas at CEUS. Although they may be visible in all three vascular phases, injuries appear more evident during the venous phase. In the later phase, the images deteriorate very quickly, and the abnormalities become indistinguishable. The venous phase is thus undoubtedly the most efficient for liver injury detection and has been called “the homogeneous phase.”
Some injuries, mainly in the liver, may appear quite large on CECT and smaller on CEUS, as reported by McGahan and colleagues. Although surgical correlation is lacking due to the conservative treatment, it is plausible that the hypoechoic area seen with CEUS is related to the parenchymal laceration and the larger area seen with CECT is the sum of the edema and the laceration. If this hypothesis is correct, this is not a pitfall but an added value of CEUS, capable of distinguishing the true lesion (laceration) from the surrounding edema. Minor lesions not seen with CEUS may be areas of edema visible only with CECT but without clinical implication.
In liver injuries, CEUS can have some drawbacks. Because of the use of low-emission-frequency harmonics, there is loss in spatial resolution and overall image quality. The poor signal arising from the most deeply located lesions may give them partially or completely unrecognized, resulting in a false-negative study. Moreover, hepatic steatosis or fibrosis increases attenuation of the US beam reducing CEUS capability and newly resulting in a false-negative study when exploring deep liver portions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







