Morbidity and mortality are high for children who present with stroke in the context of critical illness.
Morbidity and mortality are high for children who present with stroke in the context of critical illness. In emergency stroke care, time is brain: any delays in appropriate interventions compromise tissue viability.
In emergency stroke care, time is brain: any delays in appropriate interventions compromise tissue viability. Emergency neuroimaging, ideally magnetic resonance imaging if time and the emergency permit, in order to define the diagnosis and to direct diagnosis-specific intervention is good practice.
Emergency neuroimaging, ideally magnetic resonance imaging if time and the emergency permit, in order to define the diagnosis and to direct diagnosis-specific intervention is good practice. with critical illness (8). Already among the top 10 causes of childhood death, the prevalence is probably increasing as a consequence of increased recognition and improved sensitivity of diagnostic neuroimaging using MRI or cranial computed
with critical illness (8). Already among the top 10 causes of childhood death, the prevalence is probably increasing as a consequence of increased recognition and improved sensitivity of diagnostic neuroimaging using MRI or cranial computed  tomography (CT) angiography. In addition, therapeutic advances that now allow children with predisposing conditions such as SCD, CHD, and malignancy to survive may unfortunately increase the risk of stroke.
tomography (CT) angiography. In addition, therapeutic advances that now allow children with predisposing conditions such as SCD, CHD, and malignancy to survive may unfortunately increase the risk of stroke.may be less than in adults because of the lack of proven acute interventions, but the long-term healthcare costs of rehabilitation and continued care and treatment of recurrent stroke might well be greater (9,10).
TABLE 64.1 DIFFERENTIAL DIAGNOSIS IN CHILDREN WHO PRESENT WITH ACUTE FOCAL NEUROLOGIC DEFICIT | |
|---|---|
|
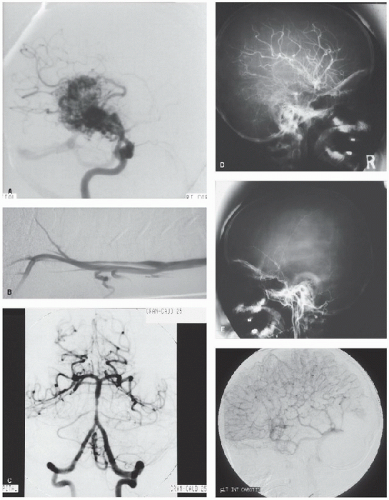
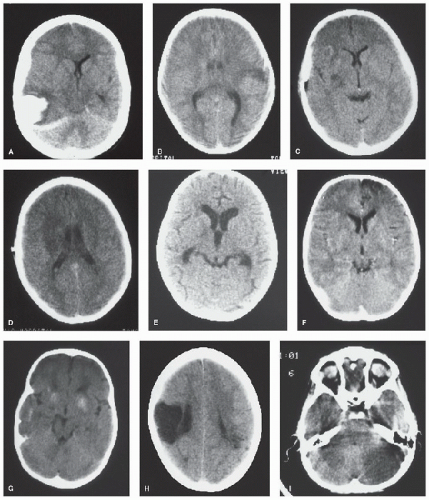 FIGURE 64.1. CT scans from children with hemorrhagic and ischemic (arterial and venous) stroke and its mimics. A: Spontaneous intracerebral hemorrhage with midline shift. B: Cortical ischemic stroke after minor head injury. C: Small infarct associated with middle cerebral artery stenosis. D: Larger infarct after recurrence of stroke in C. E: Hydrocephalus and basal ganglia infarct in tuberculous meningitis. F: Frontal infarction associated with moyamoya. G: Calcification associated with moyamoya. H: Old congenital infarct. I: Cerebellar infarction in an unconscious boy. (Continued). |
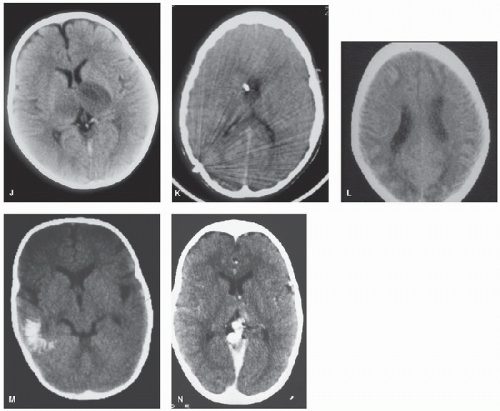 FIGURE 64.1. (Continued). J: Bilateral thalamic infarction in a 4-year-old girl with severe iron deficiency anemia and sinovenous thrombosis. K: Cerebral edema and dense straight sinus thrombosis in sickle cell disease. L: Bilateral watershed ischemia secondary to rapid blood pressure reduction in severe hypertension. M: Calcification of a parieto-occipital pial angioma in Sturge-Weber syndrome. N: Vein of Galen malformation (contrast CT). |
as an important cause of arterial vascular disease and infarction. Homozygosity for the thermolabile variant of the methylene tetrahydrofolate reductase gene appears to be a risk factor for neonatal and childhood AIS, sinovenous thrombosis, and recurrence in childhood AIS (27). Homocysteine levels are influenced by diet; supplementation of folate, vitamin B12, and vitamin B6 may reduce plasma homocysteine levels, although few available data support efficacy in stroke prevention, and a varied, healthy diet should provide adequate intake.
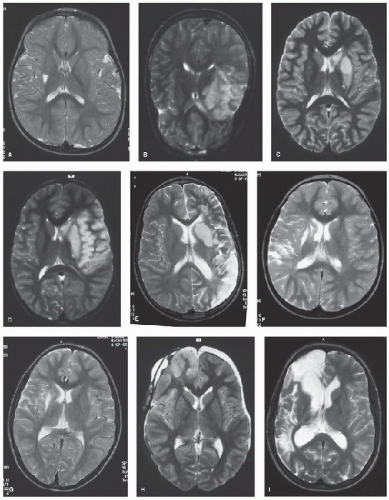 FIGURE 64.2. MRI scans from children with first and recurrent ischemic (arterial and venous) stroke and its mimics. a: Basal ganglia infarct associated with transient cerebral arteriopathy after varicella. b: Temporal infarction associated with dissection. c: Small infarct associated with middle cerebral artery stenosis. d: Larger infarct after recurrence of stroke in c. e: Recurrent infarction after dissection. F: “Silent” posterior watershed recurrent infarction after embolic occlusion. g: Deep white matter infarct in Haemophilus influenzae meningitis. h: Right frontal cortical edema after craniopharyngioma surgery. i: “Silent” recurrent infarction in the posterior watershed territory in the same patient as in h. (Continued). |
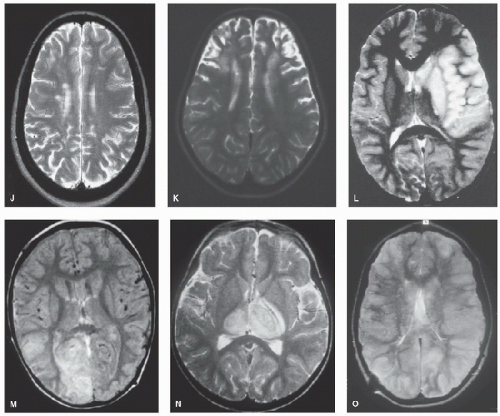 Figure 64.2. (Continued). J: “Silent” infarction in the deep white matter in sickle cell anemia. k: Bilateral frontal infarction in sickle cell anemia and moyamoya. L: Large middle cerebral artery territory infarct in sickle cell anemia. M: High signal in the right occipital lobe associated with straight sinus occlusion; subacute thrombus is seen as high signal on proton density images. N: Bilateral thalamic infarction in sinovenous thrombosis (same patient as Fig. 64.1J). O: Thrombus in the straight and left transverse sinuses on coronal T1-weighted MRI. |
factor) (45) and molecules on the red blood cells and endothelial cell surfaces (e.g., vascular cell adhesion molecule).
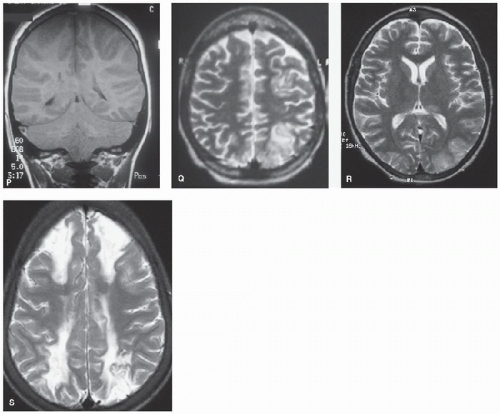 FIGURE 64.2. (Continued). P: Widespread cortical and basal ganglia hypersignal on T2-weighted axial MRI (same patient with sickle cell disease as shown in Fig. 64.1K). Q: T1-weighted coronal images showing marked swelling of the cerebral hemispheres and posterior fossa leading to tonsillar descent and brain death in same patient with sickle cell disease as shown in Figs. 64.1K and 64.2P. High signal is seen in the right transverse sinus (delta sign) due to sinovenous thrombus. R: Occipital edema in distribution compatible with reversible posterior leukoencephalopathy in sickle cell anemia and acute chest crisis. S: Bilateral watershed infarction in sickle cell anemia and facial infection. |
difficult to distinguish from transient cerebral arteriopathy (51). Approximately 80% of posterior circulation dissections are extracranial, and more than half are located within the vertebral artery at the level C1-C2 (52). Multiple dissections are common and are seen in 8%-28% of children.
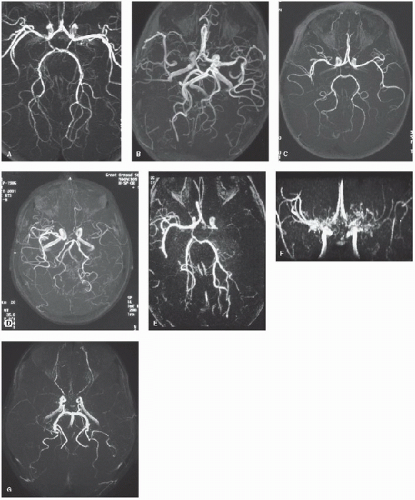 Figure 64.3. MRA from children with first and recurrent arterial ischemic stroke. a: “Transient” cerebral arteriopathy associated with basal ganglia infarct (Fig. 64.2a). b: Progressive arteriopathy associated with falloff in cognitive performance after Haemophilus influenzae meningitis and deep white matter infarction (Fig. 64.2g). c: Persistence of embolic arterial abnormality associated with “silent” recurrent infarction (Fig. 64.2F) in the posterior watershed territory. d: Unilateral arteriopathy associated with recurrent transient ischemic attacks in sickle cell anemia. e: Unilateral occlusion at the origin of the middle cerebral artery in sickle cell anemia with a large middle cerebral artery territory infarct (Fig. 64.2l). F: Bilateral moyamoya collaterals associated with severe stenosis of the distal internal carotid/proximal middle cerebral arteries. g: Bilaterally reduced flow in the middle cerebral artery without obvious moyamoya collaterals in sickle cell disease. |
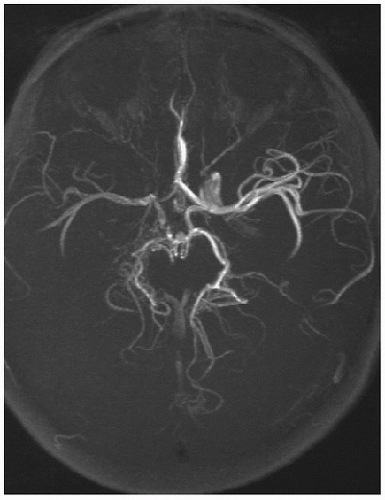 FIGURE 64.5. Axial fat-saturated, T1-weighted MRI of the neck in a patient presenting with an acute hemiparesis shows blood in the wall characteristic of dissection. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree