Relatively contraindicated in pt’s with carotid bruits, stenosis, or aneurysm. The right side is better as can access (“straight shot”) R atrium w/o encountering major angles. Avoid if pt has SVC syndrome, local infection or unable to tolerate the Trendelenburg position. Although bleeding d/o’s are relative contraindications to central venous cannulation, the IJ approach is preferred over the subclavian route, as the IJ site is compressible.
Advantage: less risk of pleural puncture (PTX), easy to compress a hematoma, easy landmarks.
Disadvantages: difficult to cannulate if hypovolemic, a “blind” puncture, risk of carotid injury, restriction of the pt’s neck mobility. Not a good site if pt has tracheostomy as insertion site may be exposed to infected stomal secretions. Complications |
Anatomy: When the head is turned to the opposite side, the vein runs from a straight line from the pinna (of the ear) to the lateral SC-joint. It is positioned behind the sternocleidomastoid muscle lateral to the carotid artery. Grab the SCM and follow up the lateral border of the medial head. The catheter should be placed at a location at the upper confluence of the two bellies of the sternocleidomastoid (apex of the triangle), at the level of the cricoid cartilage.
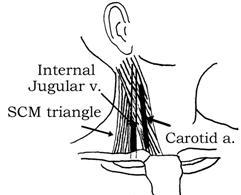
Near the base of the neck it joins the carotid a. within the sheath to become the most lateral structure in the carotid sheath. The IJ vein, the internal (and, later, the common) carotid artery, and the vagus nerve course together in the carotid sheath. The IJ vein occupies the anterior lateral position in the carotid sheath. The IJ vein emerges from under the apex of the triangle of the 2 heads of the sternocleidomastoid muscle and joins the subclavian vein behind the clavicle. As the vein approaches its supraclavicular junction with the subclavian vein, it assumes a more medial position in the triangle formed by the 2 heads of the sternocleidomastoid muscles.
Step #1: Place the pt in 10-25 deg of Trendelenburg (head down) position (to distend the neck veins and to prevent an air embolism). Choose a location on the right or left. If lung function is symmetrical and no chest tubes are in place, the right side is preferred because of the direct path to the superior vena cava. Prepare the skin with Betadine solution using sterile technique and a drape. Infiltrate the skin and deeper tissues with 1% lidocaine.
• No need to turn the head with cannulating the IJ according to a RCT on 1332 pt’s (mean age 53), cannulation times, success rates, and complication rates were similar with neutral head position and 45-degree head rotation (Anesth Analg 2012;114:777)……Complications (e.g., carotid artery puncture, neck hematoma, multiple skin or vein punctures, and difficult guidewire insertion) occurred in 13% of cannulations in each group. Risk for complications was associated with smaller vein diameter, greater vein depth, anterolateral or lateral vein position, female sex, American Society of Anesthesiologists physical status 2, and body-mass index >30 kg/m2…..Carotid artery puncture accounted for <1% of complications; no pneumothoraces or hemothoraces were noted. A vein that is large, superficial, and anteriorly positioned is your best bet.
Step #2: 3 main approaches: (ant, post, central) All use the same landmarks, but differ in entry site and needle angle. Gently palpate & retract the carotid medially as you insert the needle. While aspirating, advance the 22g scout (finder) needle (3ml syringe) until the vein is located and blood flows back into the syringe. If use U/S can skip the scout needle.
Central / Middle approach –> Has the lowest risk of carotid cannulation (safest). Skin puncture is at the apex of the triangle formed by the two bellies of the SCM and the clavicle (~3 fingers lateral & 3 fingers sup to sternal notch). Use 3 fingers of the nondominant hand to lightly palpate & medially retract the carotid artery. Needle enters at 45-60 deg angle to the skin in the frontal plane and directed toward the ipsilateral nipple using the dominant hand and keeping constant negative pressure in the syringe. Usually hit the IJ in 2-4cm. If no initial return, redirect the needle 1-3cm more laterally using the same puncture site, if still unsuccessful, then try 1cm medially. Once get dark blood freely flowing into the syringe, rotate the needle 360 deg while aspirating to that the bevel is completely in the vein. Confirm that it is just an ooze and not a pulsating artery (if unsure send off an AGB or hoop up a CVP monitor).
Anterior approach –> Enter at the midpoint of the sternal head of the SCM, ~5cm from both the angle of the mandible and the sternum. Palpate the carotid and enter at 30-45 deg angle 1cm lateral and parallel (directed caudally and toward ipsilateral nipple). Hit IJ in 2-4cm.
Posterior approach –> Enter 1cm dorsal to where the EJ crosses the posterior border of the clavicular head of the SCM (~5cm cephalad of the clavicle). Direct needle caudally toward the sternal notch at 45 deg angle. Hit IJ in 5-7cm.
Step #3: Now can either remove the finer “scout” needle and advance a 16-gauge, thin wall catheter-over- needle (directly above it) with an attached syringe along the same path as the scout needle. Or remove the finer needle and introduce the larger one in the same plane. When back flow of blood is noted into the syringe, advance the catheter into the vein. Remove the needle and confirm back flow of blood through the catheter and into the syringe. Remove the syringe, and use a finger to cover the catheter hub to prevent air embolization. Some kits have syringes with a “closed” system (can insert guidewire through the middle of the syringe).
Step #4: With the 16 g catheter in position, advance a spring guide wire (0.89 mm x 45 cm) through the catheter. The guidewire should advance easily without resistance. Always hold onto the guidewire. With the guidewire in position, remove the catheter and use a # 11 scalpel blade to nick the skin adjacent to the wire. Keep the needle/ syringe firmly in place as manipulate the guidewire by creating a “tripod” with your fingers.
Step #5: Either first place the dilator over the wire (insert with a twisting movement) or directly insert central vein catheter over the wire, holding the wire secure at all times. Once the wire is thru the distal port, pass the catheter into the vein, remove the guidewire, and suture the catheter with 0 silk suture, tape, and connect it to an IV infusion. Auscultate the lungs. Obtain a CXR to rule out PTX and confirm position of the catheter.
Notes: In anesthetized surgical patients, the incidence of localized hematomas was lower (10% vs. 4% = bevel-up vs bevel-down) when the puncture needle was inserted with the bevel down than with the bevel up into the internal jugular vein (IJV) (Crit Care Med 2011;Oct 6;e-pub ahead of print)……Although the bevel-up position for insertion of IJV catheterization needles generally is recommended and makes intuitive sense, this study suggests that using either the bevel-up or the bevel-down position is reasonable.
Subclavian Vein:
It is a continuation of the axillary vein as it passes over the 1st rib. The pleura is just 5mm deep to it at its point of origin (1-2% risk of PTX).
Advantage: remains open even with profound circulatory collapse. Less restricting to the pt. Good choice in emergency situation as placement will not interfere with airway management. A coagulation d/o is generally not a contra.
Disadvantage: Has increased risk of PTX in pt’s with emphysema or bullae as the pleural space is easily entered with the “blind” stick. Difficult to apply pressure if the artery becomes punctured. It is located in the angle formed by the med 1/3 of the clavicle and the first rib. Complications | Equipment & Basic Anatomy |
Step #1: Position the pt supine in slight Trendelenburg. Prepare the area with Betadine X3, using sterile technique, drape the area and infiltrate 1% lidocaine into the skin and  tissues. In the past it has bee advocated to arch the shoulders by placing a rolled towel located between the pt’s scapulae, and turning the pt’s head towards the contralateral side, BUT this actually reduces the SC veins target size as measured by Duplex U/S (Arch Gen Surg 2003;138:96-1000).
tissues. In the past it has bee advocated to arch the shoulders by placing a rolled towel located between the pt’s scapulae, and turning the pt’s head towards the contralateral side, BUT this actually reduces the SC veins target size as measured by Duplex U/S (Arch Gen Surg 2003;138:96-1000).
Step #2: The needle enters at the tubercle of the clavicle, palpated on the inferior surface ~1/3 to 1/2 the length of the clavicle form the sternum. Depress under the clavicle distal to this area with your thumb as your pointer finger rest at the angle of Louis. Advance the 16-gauge catheter-over-needle, with syringe attached until the clavicle bone and needle come in contact.
Step #3: Slowly probe (walk) down with the needle until the needle slips under the clavicle, and advance it slowly (should feel no resistance) towards the finger resting on the sternal notch at a 25 deg angle to the thorax (parallel to the bed). Keep bevel pointed inferomedial to encourage the guidewire to enter the innominate vein. Advance until see a back flow of venous blood enters the syringe. Remove the syringe, and cover the catheter hub with a finger to prevent air embolization. Ask the pt to hum or hold breath as you pull the needle out and cover it.
Step #4: With the 16-gauge catheter in position, advance a 0.89mm x 45cm spring guide wire through the catheter. The guide wire should advance easily without resistance. With the guide wire in position, remove the catheter, and use a #11 scalpel blade to nick the skin.
Step #5: Place the central line catheter over the wire, holding the wire secure at all times. Pass the catheter into the vein, and suture the catheter with 2-0 silk suture, tape, and connect to an IV infusion. Auscultate the lungs. Check CXR to confirm position and rule out PTX.
Pulmonary Artery Catheterization (PAC):
Link: PA Cath Patterns:
Indications: need to asses the adequacy of the intravascular volume (CVP cath may be inadequate in the face of pulmonary dysfunction): oliguria, hypotension, high risk surgical candidate. To assess the cause and tx of pulmonary edema (high vs. low wedge). To assess therapy for shock: hypovolemia, septic or cardiogenic). To assess effects of tx on O2 delivery. Being used less nowadays as it is an expensive, invasive procedure and a number of randomized trial suggest that routine placement of PA catheters is not beneficial in a defined category of pt’s.
Insertion sites: subclavian, antecubital, femoral, axillary. The right IJ offers a straights pathway to the right atrium, the left subclavian approach takes advantage of the curve in the PAC from its factory packaging.
Contra: none are absolute. Preexisting site infection, known or suspected anatomic abnormality, coagulopathy, LBBB, pulmonic stenosis, pacemaker wires. Complications | Equipment & Basic Anatomy |
Step #1: Using sterile technique, cannulate a central vein using one of the above techniques.
Step #2: Advance a guide wire through the cannula, then remove the cannula, but leave the guide wire in place. Keep the guide wire under control at all times. Nick the skin with a number #11 scalpel blade adjacent to the guide wire, and pass a number 8 French introducer over the wire into the vein. Remove the wire and connect the introducer to an IV fluid infusion, and suture with 2-0 silk.
Step #3: Pass the proximal end of the pulmonary artery catheter (Swan Ganz) to an assistant for connection to a continuous flush transducer system.
Step #4: Flush the distal and proximal ports with heparin solution, remove all bubbles, and check balloon integrity by inflating 2ml of air. Check pressure transducer by quickly moving the distal tip and watching monitor for response.
Step #5: An introducer sheath is put in place before the PAC. Pass the catheter through the introducer into the vein, then inflate the balloon with 1.0ml of air one passed the introducer, and advance the catheter until the balloon is in or near the right atrium. The catheter is marked at 10cm intervals to aid in determining the depth of insertion.
Step #6: The approximate distance to the entrance of the right atrium is determined from the site of insertion: Right IJ vein –> 10-15 cm. Subclavian vein –> 10 cm. Femoral vein –> 35-45 cm.
Step #7: Advance the inflated balloon, while monitoring pressures and wave forms as the PA catheter is advanced. Watch for ventricular ectopy during insertion. Advance the catheter through the right ventricle into the main pulmonary artery until the catheter enters a distal branch of the pulmonary artery and is stopped (as evidenced by a pulmonary wedge pressure waveform). Link: PA Cath Patterns:
Other: Do not advance catheter while the balloon is deflated, and do not withdraw the catheter with the balloon inflated. The balloon acts as a spinnaker sail that is carried by the blood through the venous system. After placement, obtain a chest X-ray to ensure that the tip of catheter is no farther than 3-5 cm from the mid-line, and no PTX is present. It shouldn’t take >1ml to wedge. Measures pulmonary venous pressure ~= L atrial pressure ~= LV end-diastolic pressure, false readings if have mitral stenosis. Also uses a Thermaster to determine cardiac parameters via temperature dilution. Give a bolus of fluid to R atrium (CVP port) at room temp, simultaneously measure temp in pulmonary artery (tip), there is a smaller temp change with incr cardiac output. False readings with TR and VSD.
Zeroing and Leveling: Leveling a system is placing the transducer at the level of the phlebostatic axis so that the vascular pressures are referenced to the pressure of the left atrium. The phlebostatic axis is defined as the intersection of a line extending along the lateral aspect of the thoracic cage at the level of the 4th intercostal space and a horizontal line midway between the most anterior and posterior aspects of the chest. The transducer is often either taped to the pt or the bed at the phlebostatic axis in a supine pt. The system is typically zeroed once per nursing shift, some zero it before each measurement. A good waveform is essential for accurate measurements, checking the square wave test assesses the integrity of the monitoring system: the catheter is flushed under pressure (300mmHg), the waveform rises rapidly exceeding the scaled upper pressure limit, as the flush is released, the waveform is observed — it should quickly return to 0 and actually overshoot and oscillate before resuming the typical transduced pressure waveform – these oscillations should not be spaced further apart than 1 little box (1mm) at 25mm/d or 2 little boxes (2mm) at 50 mm/d.
The “frequency response” is calculated as cycles/s = paper speed / distance between the consecutive oscillatory peaks. Next, a graph is used to check that the “dampening coefficient” is optimal (ranges between 0.3-0.75).
Info: Clinical management involving the early use of PAC in pt’s with ARDS, shock or both did not significantly affect mortality (JAMA 2003;290:2713-20).
• Therapy in elderly, high-risk surgical pt’s guided by PA catheters was better than standard care (central venous catheter) (NEJM 2003;348:5-14).
• PAC was associated with increased mortality in pt’s with acute coronary syndromes, except in pt’s with cardiogenic shock (Am J Med 2005;118:482-88).
• A RCT with 1041 adult pt’s reinforces the conclusions of previous studies that PAC neither increases nor decreases short-term mortality rates (Lancet 2005;366:472-7). PACs do not improve outcomes for pt’s hospitalized for recurrent severe heart failure (JAMA. 2005;294:1625-1633, 1664-1670) (433 pt’s at 26 sites)…likely with be relegated to salvage therapy of an extremely small and select number of pt’s.
• PACs are associated with more complications and do not improve outcomes over fluid management based on a CVP protocol in pt’s with acute lung injury (NEJM Posted online May 21, 2006). Findings from the ongoing Fluid And Catheter Therapy Trial (FACTT) indicate that a pulmonary artery catheter has no survival advantages and no benefit for ICU length of stay or healthcare utilization compared with a CVP in monitoring the status of pt’s with ARDS (SCCM 36th Annual Meeting: Presented February 19, 2007).
**Ref:(Irwin and Rippe’s Intensive Care Medicine, 6th ed., 2007, Lippincott-Raven) (Infections related to central venous catheters. Mayo Clin Proc. 1990;65:979-86) (Intravenous and central catheter infections. Surg Clin North Am. 1994;74:557-70) (Central venous catheter placement and complications. Crit Care Med. 1994;22:1516-18) (Arterial cannulation: how to do it. Br J Hosp Med 1997;57:497-9) (Arterial cannulation. Anesthesia 1995;50:576) (The pulmonary artery catheter in the ICU. J Crit Illness 2003;18:9-18) (Prevention complication of central venous catheterization. NEJM 2003;348:1123-33)
Normal Pulmonary Artery Catheter & Cardiac Parameters:
Link: Fick Principle | PA Cath Patterns | Conditions / Hemodynamic Parameters |
Directly measured and most important are the CO, PAP and PCWP.
Measured Parameters:
Central Venous Pressure (CVP = Right Atrial Pressure): 0-8mmHg. RV preload. If >11 and in CHF give 80mg Lasix q8hr.
Pulmonary Artery Pressure (PAP) Mean: 9-20 mmHg.
Pulmonary artery SBP (PAS): PAP Systolic: 15-30 mmHg. >35 = pulmonary HTN.
Pulmonary artery DBP (PAD): PAP Diastolic: 4-15 mmHg. Should be a points above the PCWP, can be used instead of a wedge.
Pulmonary Cap Wedge Press (PCWP): 6-12 mmHg (~16 with AMI). PCWP is the dampened LA pressure (indirect measure of LA pressure) which reflects LVEDP, which reflects LVEDV. Preload of LV, a hydrostatic gradient. PCWP of 15-15 leads to DOE with dilated upper lobe veins on CXR, @ 25-35mmHg leads to dyspnea at rest, orthopnea, interstitial edema & Kerley B lines on CXR.
If >35 acutely get pulmonary edema. Not a valid measurement when intrathoracic pressure exceeds the distending pressure of the pulmonary capillary bed (parenchymal lung dz, severe volume depletion, pulmonary vascular dz, catheter tip malposition).
Pearls: the diastolic pressures will be = in all 4 chamber with pericardial tamponade or constrictive pericarditis. Normally the diastolic PA pressure = PCWP (except with pulmonary HTN). If have an inferior AMI and see a decr CO & PCWP with incr RA pressure you have a RV infarction (check R-side ECG). If have decr CO with incr PCWP and RA pressure then have biventricular failure due to cardiogenic shock.
Right Atrial (RA) pressure: 1-7 mmHg, if elevated, then suggests RV failure, should see JVD. The mean RA pressure is a measure of both the hydrostatic pressure on the systemic veins & RV end-diastolic (filling) pressure.
RV Pressure (RVP) Systolic: 10/-30 mmHg.
RVP Diastolic: 0-8 mmHg = RV end-diastole.
LV Systolic Pressure: 100-140 mHg.
LV End-diastolic: 3-12 mmHg.
Aortic Systolic @ 100-140, diastolic @ 60-90, mean @ 70-105.
Fick Principle: the uptake of a substance by an organ is a product of blood flow and the arteriovenous (A-V) substance difference across the organ. For CO = 100 X O2 Consumption / (arterial O2 content – venous O2 content).
The blood O2 content (Vol %) is calculated from the saturation % X Hemoglobin (g/dL) X 1.36. Venous blood is from the PA, arterial blood is from the periphery.
Cardiac Output (CO): HR X stroke volume. Nl = 3.5-8 L/min. Total body perfusion.
CO l/min = 125 mL O2 /min/M 2 x 100 = 8.5 {(1.36)(Hb)(SaO2 ) – (1.36)(Hb)(SvO2)}. Measured by either Fick method or thermodilution (invalid with tricuspid regurge). Usually increased in septic shock, cirrhosis hyperthermia, thyrotoxicosis, Paget’s dz or a large AV fistula. Low in cardiac failure, massive PE, cardiac tamponade, hypovolemia, constrictive pericarditis, tension PTX or high values of PEEP.
Remember that Cardiac Output is not the Same Thing as Ejection Fraction: Cardiac output equals the volume of blood pumped by the heart per minute, whereas stroke volume (SV) is the amount pumped on a single beat. Cardiac output can be determined using indicator dilution methods (Fick, thermodilution), Doppler velocity data, ventricular impedance, and radionucleotide methods. Ejection fraction (EF) is measured in percent: EF (%) = (SV/EDV) × 100%; whereas SV is calculated as SV = EDV – ESV, with EDV the end-diastolic volume and ESV the end-systolic volume. Ejection fraction is typically estimated using qualitative two-dimensional echocardiography. However, the accuracy depends on the observer experience. Inadequate definition of the endocardial border and regional wall motion abnormalities with asymmetric ventricular contraction may lead to inaccuracies, which may be overcome by various yet time-consuming ventricular volume formulas. Although a satisfactory ejection fraction would suggest a similar adequate cardiac output, this relationship does not always hold true. In mitral regurgitation, ejection fraction can be excellent, yet part of the left ventricular end-diastolic volume is ejected back into the left atrium via the incompetent mitral valve during ventricular systole. In this scenario, ejection fraction can be normal, yet cardiac output is diminished. In contrast, cardiac output is supranormal in aortic incompetence, but part of the forward stroke volume regurgitates back into the left ventricle during ventricular diastole. As the disease progresses, heart failure will develop with a reduction of ejection fraction pseudonormalizing cardiac output, eventually reaching a point at which both hemodynamic parameters are severely depressed. Regurgitant volume and regurgitant fraction are used in grading the severity of these valvular lesions, adding more valuable information to the assessment of cardiac function. Temperature thermodilution methods for cardiac output determination remain accurate because these are typically measured in the pulmonary artery using a Swan-Ganz catheter and right-sided cardiac output must equal left-sided cardiac output.
Stroke Volume: CO / HR = 55-100 mL.
Stroke Index: 30-65 ml/beat/m2.
Cardiac Index (CI): CO/ BSA. Nl = 2-4 L/min/m2.
Double Product: (HRXSBP)/ 100. Nl 60-140, relative myocardial O2 use.
Mean arterial Press (MAP): (SBP + 2DBP) / 3 or [(SBP — DBP)/3] + DBP. = 85-95mmHg. End organ perfusion.
Systemic Vascular Resistance (SVR): [ 80 X (MAP – CVP)] / CO. Nl = 700-1600 dynes/sec/cm-5. Geometry of systemic arterioles. Calculated value, need to take with a grain of salt.
SVR Index (SVRI): = SVR X BSA (in m2). Normal SVRI = 2130 +450 dyn/d/cm-5/m2.
Left Ventricular Stroke Volume (LVSV): range of 70-94 ml.
LVSVI (Index): range of 30-65 mL/m2.
Total Pulmonary Vascular Resistance (PVR): [ 80 X (MPAP — PCWP)] / CO. = [(PA – PCW) X 80] / CO. Normal is ~1/6 the SVR = 100-300 dyn/sec/cm-5. Pulmonary Vascular Resistance is 20-130. Geometry of pulmonary arterioles. It is elevated in shunts, myocardial dz, pulmonary vasculature obstruction, hypoxemia, toxins and primary pulmonary HTN.
PVRI = 80 X (mean PAP — PAWP) / CI = 80-240 dynes-sec/cm2/m2.
Arterial O2 Content (CaCO2): (Hb X 1.36) SaO2 + (PaO2 X 0.0031) = 16-22 ml O2/ dL blood or vol%.
Mixed Venous O2 content (CvO2): (Hb X 1.36) SvO2 + (PvO2 X 0.0031) = 12-17 mL O2/dL blood. A measurement of overall tissue oxygenation extraction. Calculated using the Fick equation after measuring the mixed venous saturation.
Mixed Venous O2 Saturation = SVO2: Can be useful in evaluating hypoxemia in the critically ill. May be decreased with hypoxemia, hypovolemia or anemia. May be elevated in sepsis. Serial lactate levels (reflects anaerobic metabolism) can help monitor the pt’s response to therapy (Evaluating hypoxemia. J Crit Care Illness 2005;20:90-C3). Can be measured with a specially equipped catheter (within a PA cath) that contains fiberoptic bundles that transmit and receive light to give readings of the pt’s venous blood O2 content. Also affected by cardiac output, O2 consumption and SaO2.
Mixed Venous O2 sat (Pulmonary artery): 75%.
Arterial oxygen capacity =(Hg(gm)/100 mL) x 1.36 mL O2 /gm Hg. O2 carrying capacity is mostly due to Hb as O2 SATs have little affect.
DO2: O2 delivery: CaO2 X CO X 10. nl = 640-1400 mL/min.
VO2: O2 uptake: C(a-v)O2 X CO X 10. Nl = 180-280 mL/min. The best hemodynamic parameter to asses if shock is present.
Oxygen Consumption Index (VO2I): range of 113-148 ml/min X m2.
Arteriovenous O2 Difference: 30-50 ml/L.
Gas Equations:
A-a gradient: PAO2 – PaO2, Normal=[(age / 4) + 4].
Alveolar Gas Equation: PAO2 = (FiO2 x 713) – (PaCO2 / RQ).
RQ = VCO2 / VO2.
Bohr Equation: VD/VT = (PaCO2 – PETCO2)/PaCO2, Normal 0.3.
PAO2 [alveolar O2 pressure].
PaO2 [arterial O2 pressure, normal=(100 – (age / 3))].
RQ [normal respiratory quotient=0.8)].
VT [tidal volume]
VD [dead space]
PECO2 [exhaled PCO2]
PETCO2 [end tidal CO2]
TISSUE OXYGENATION EQUATIONS
CaO2 = (Hb x SaO2 x 1.34) + (PaO2 x 0.003) x 10.
O2 capacity of Hb = 1.34 ml O2 / gm Hb.
VO2 = CO x 13.4 x Hb x (SaO2 – SvO2), normal=[110 – 160].
VO2 = CO x (CaO2 – CvO2).
DO2 = CO x 13.4 x Hb x SaO2, normal=[520 – 570].
DO2 = CO x CaO2.
O2ER = (VO2 / DO2) x 100, normal=[20 – 30%].
O2ER = ((SaO2 – SvO2) / SaO2) x 100.
SvO2 = (CO x Hb x SaO2) / VO2, normal=[65 – 75%].
DO2 [arterial delivery (ml / min)].
VO2 [tissue uptake (ml / min)].
VCO2 [CO2 elimination (ml / min)].
Normal adult VO2 (oxygen consumption) = 3 mg/kg/min.
Normal infant VO2 (oxygen consumption) = 7 mg/kg/min.
SHUNT EQUATION
QS/QT = (CcO2-CaO2)/(CcO2-CvO2) normal is 5%.
Links: Conditions / Hemodynamic Parameters | Swan Orders | Central Venous Pressure (CVP) | Shock | Pulmonary & Cardiac Parameters & Formulas | PA Cath Placement |
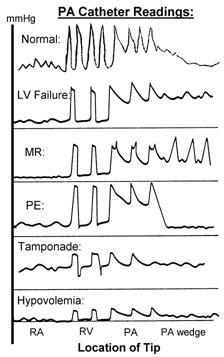
Swan-Ganz Information: As obtain readings from the PA catheter, you go from RA to RV to PA to PCW (see illustration).
Measured: CVP. RV pressures. PA systolic. PA diastolic. PCWP (pulm art occlusion pressure = PAOP). Cardiac output (via thermal dilution).
Calculated: Mean PA pressure. PVR. SVR. SVR = (MAP-CVP) SO/CO
PCWP = Wedge pressure = Pulmonary artery wedge pressure (PAWP = LAP, L atrial pressure, in mm Hg, pulmonary artery diastolic pressure may be used instead).
• Wedge goal is 18. (if high then diurese, if low then fluid bolus) If using PEEP it will raise this so you need to take 1/3 of the PEEP and subtract it to get the actual wedge. Left Atrial Pressure = PCWP – (1/3 PEEP).
Cardiac Index (CI) = CO/BSA. Goal is to get above 2.2. If low, add inotropes.
Pulmonary artery pressure (PAP = MAP).
Central Venous Pressure (CVP = RAP, R atrial pressure).
Systemic Vascular Resistance (SVR): multiply by 80 to give SI units of dynes/sec/cm-5, normal = 1000 dyn). Almost all above conditions will have incr HR, decr venous compliance (except septic shock), decr CaO2 and decr DO2.
Elevated R atrial pressure (RAP): volume overload, RV failure, TR, TS, cardiac tamponade, constrictive pericarditis, chronic LV failure, PEEP >10 cm H2O.
Elevated pulmonary artery occlusion pressure (PAOP): volume overload, LV failure, MR, MS, cardiac tamponade, overwedged PAC, PAOP measured in zone 1 or 2, PEEP >10.
Low RAP or PAOP: hypovolemia.
Atrial waveforms: the RA waveform is a composite of 3 risings (a,c and v) and 2 descending (x,y) waveforms. A wave = atrial contraction ((PR interval on ECG), c = tricuspid closure (in the RST portion of the ECG), v = ventricular contraction (at the termination of the T wave on ECG), x descent = atrial relaxation, y = rapid atrial emptying into the ventricle.
Large a waves: TS or MS, decreased ventricular or atrial compliance. Increased afterload, VSD.
Large v waves: TR, MR, ventricular ischemia or failure, decr atrial compliance, incr afterload, VSD.
Absent a waves: A-fib/flutter, junctional, paced of ventricular dysrhythmias.
Absent y descents: cardiac tamponade.
Exaggerated y descents: constrictive pericarditis, restrictive cardiomyopathy.
Link: PA Cath Patterns |
PCWP/ CI/ SVR/ Other:
Normal –> 6-12/ 3.5L/ 11-18/ PAP 14, CVP 5-10.
Hypovolemia –> decr/ decr/ Nl-incr/ Pt is dry. Low PCWP. Low CO. high SVR.
LV Failure –> incr/ decr/ incr.
Primary RV Failure (RV AMI) –> Nl-decr/ decr/ Nl/ decr RA press, steep Y-descent, RV diastolic dip and plateau.
Secondary RV Failure (LV Failure) –> nl-incr/ decr/ Nl-incr/ incr PVR, incr RA.
Tamponade –> incr/ decr/ incr/ RA=wedge, decr y-descent, incr x-descent, pulsus paradoxus always present.
Constrictive Pericarditis –> incr/ nl- decr/ Nl/M or W shape JVP, steep y-descent, Kussmaul’s, pulsus paradoxus in 1/3.
Acute MR –> incr, peaked v-wave so/decr/incr/Post MI, tall V-waves.
Acute VSD –> incr/ decr/ incr /Post MI, O2 step up from RA to RV to PA.
Sepsis –> decr/ nl- incr/ Nl- decr/ +F/C, Blood Cx. Normal PCWP./ Low CO and high S
ARDS –> Nl-decr /incr/ decr/ Incr / decr/ Pulmonary infiltrates with progressive hypoxemia.
Massive PE –> Nl/ decr/ incr / incr PA pressure.
COPD exac. –> 4/ incr/ decr/ incr CVP, PAP, CO.
Right Heart Failure / infarct: High RAP, low CI, High PVRI. CVP > PCWP. Preload dependent, avoid diuretics.
Left Heart Failure: High PCWP, low CI, High SVR.
Heart Failure: High CVP. Low CI. High SVRI. Normal VO2.
Cardiogenic Shock: High CVP. Low CI. High SVRI. Low VO2. High PCWP. Low CO. high SVR.
Various Shock States:
CO / SVR/ CVP (RAP)/ PCWP/ O2 consumption:
Hemorrhagic –> decr / incr/ decr/ decr/ incr.
Cardiogenic –> decr/ incr/ incr or decr/ incr/ incr.
Septic –> incr/ decr/ incr or decr/ incr or decr/ incr then decr.
Neurogenic –> decr/ incr or decr/ decr/ incr or decr/ incr or decr.
Hemodynamic Parameter | Normal Value |
Heart rate (HR) | 60–100 beats/min |
Stroke volume (SV = EDV – ESV) | 60–100 ml/beat, 40–70 ml/m2 |
Cardiac output (CO = HR × SV) | 4–8 L/min |
Body surface area (BSA) | 1.73 m2 (average 70-kg man) |
Cardiac index (CI = CO/BSA) | 2.6–4.2 L/min/m2 |
Stroke volume index (SVI = CI/HR) | 40–50 ml/beat/m2 |
Systolic blood pressure (SBP) | 120 mmHg |
Diastolic blood pressure (DBP) | 80 mmHg |
Pulse pressure (PP = SBP – DBP) | 40 mmHg |
Mean arterial pressure (MAP = DBP + S PP) | 70–105 mmHg |
End diastolic volume (EDV) | 70 ml/m2 |
End systolic volume (ESV) | 0–30 ml/m2 |
Ejection fraction (EF = SV/EDV) | 55–65% |
Central venous pressure (CVP = RAP = RVEDP) | 0–6 mmHg |
Right atrial pressure (RAP) | 0–6 mmHg |
Right ventricular end-diastolic pressure (RVEDP) | 0–6 mmHg |
Right ventricular systolic pressure | 15–30 mmHg |
Left atrial pressure (LAP = Left ventricular end-diastolic pressure = PCWP) | 5–12 mmHg |
Pulmonary artery systolic pressure (PAS) | 15–30 mmHg |
Pulmonary artery diastolic pressure (PAD) | 5–12 mmHg |
Mean pulmonary artery pressure (PAP) | 10–15 mmHg |
Pulmonary capillary wedge pressure (PCWP) | 5–12 mmHg |
Coronary perfusion pressure (CPP) | 60–70 mmHg |
Systemic vascular resistance (SVR) | 800–1200 ([dyne × s]/cm5) |
Arteriovenous O2 difference (AVDO2) | 30–45 ml/L |
Pulmonary vascular resistance (PVR) | 120–250 ([dyne × s]/cm5) |
Typical Swan Ganz Parameters Orders:
You can choose the no sleep option or write:
Maintain PCWP at 14-18 mmHg.
If PCWP <12-14 give 250 NS bolus and/or increase IV NS at 75-80 ml/hr.
If PCWP 14-15 give IV NS at 40 ml/hr.
If PCWP 16-18 TKO or decr IVF.
If PCWP >18-20 give Lasix 20-40 mg IV q6-8hr, if initial dose ineffective repeat in 1-2hr or write “If PCWP 1hr after Lasix is >18, call”.
If urine output (UO) <30-40 ml/hr >2hr call (if PCWP >12-15 consider Lasix. If <12 give fluids & albumen).
Central Venous Pressure (CVP):
= R atrial pressure = the pressure exerted by the blood against the walls of the intrathoracic venae cava. A means of assessing cardiac performance and guiding fluid therapy. Reflects the degree of fluid overload (or dehydration) in the absence of cardiac tamponade in pt’s without significant preexisting cardiopulmonary disease. Varies with respiration. When lying down it is 6-8 mmHg. The upper limit of normal for an ill person is 10mmHg.
Low: <6 cm H2O, rarely associated with a PCWP >12 cm H2 O in septic pt’s.
High: >12 cm H2O. CVP readings in the range of 16-18 cm H2O are typically seen in acute cardiac tamponade.
Goals: Normal: 6-12 cm H2O. When on mechanical ventilation with PEEP and needing volume, commonly use 20. If exceeds 15-18, commonly need PCWP to follow in order to precisely titrate fluids. In normal conditions the L atrial pressure is within 2-3mmHg of the R atrial pressure. Thermodilution techniques can be used to estimate the CO. Tricuspid regurgitation (TR) is seen as a prominent V wave followed by a steep y-descent. With severe TR the mean CVP is more representative of the mean RV pressure than is of central volume.
The four major indications: Acute circulatory failure. Anticipated massive blood transfusion for fluid replacement therapy. Cautious fluid replacement in pt’s with compromised cardiovascular status. Suspected cardiac tamponade.
Fluid Challenge Test: aliquots of 50-200 mL of crystalloid are sequentially administered, and measurements of CVP levels are obtained after 10 minutes. If the CVP is >5 cm of H2O over the initial measurement, the fluid challenge is discontinued, and one assumes that the right ventricle is unable to handle an additional fluid load. Increases of between 3-5 cm H2O over the initial CVP value are equivocal, and additional measurements are taken over the next 30 minutes if this reading is obtained. Increases of <2 cm over the original reading or a return of higher readings to this level within 30 minutes is indicative of volume depletion.
Measurement Technique: Although CVP may be determined with a manometry column assembled at the bedside, the most common technique in practice is measurement with an electronic transducer interfaced to a monitoring system. Typical transducers include a nipple valve attached to a pressurized bag of saline to allow easy flushing of the system. To use these manometers, the transducer is attached to the pt’s central line with a length of flexible yet fairly rigid-walled tubing filled with saline. A three-way stopcock is placed between the pt and the transducer to simplify line flushing and calibration. All air bubbles are flushed from the system by opening the stopcock to air and flushing saline through the line. Air bubbles should not be flushed into the pt. Even tiny bubbles left in the tubing will dampen the CVP wave and potentially cause underestimation of venous pressure. After the system has been flushed, the stopcock (with the transducer still open to air) is placed at the level of the pt’s tricuspid valve. The monitor detecting the transducer’s signal is then “zeroed,” or calibrated. The transducer is calibrated at the level of the tricuspid valve, which can be approximated on the skin surface as a point at the midaxillary line and fourth intercostal space. Finally, the stopcock is set so that the transducer is in continuity with the pt’s venous catheter. In spontaneously breathing pt’s, readings should be taken at the end of inspiration of a normal breath. If the pt is receiving positive-pressure ventilation, the CVP changes during the respiratory cycle are reversed, rising with inspiration and decreasing with expiration. In these pt’s, readings should be taken near the end of expiration. Thus, during both normal and mechanical ventilation, the lowest reading is a useful estimate of the mean CVP.
Faulty CVP Readings: Increased intrathoracic pressure (ventilator, straining, coughing). Reference points in error. Malposition of catheter tip. Blocking or ball-valve obstruction of catheter. Air bubbles in circuit. Readings during wrong phase of ventilation. Readings by different observers. Vasopressors (presumed).
Controlled compression sonography at the forearm can provide central venous pressure (CVP) measurements via superficial peripheral veins without the need for intravenous catheterization (J Am Coll Cardiol 2007;50:1584-1589)….presents an attractive alternative for assessment of emergency room pt’s when reliable estimation of CVP is desirable and an invasive technique is less than optimal.









