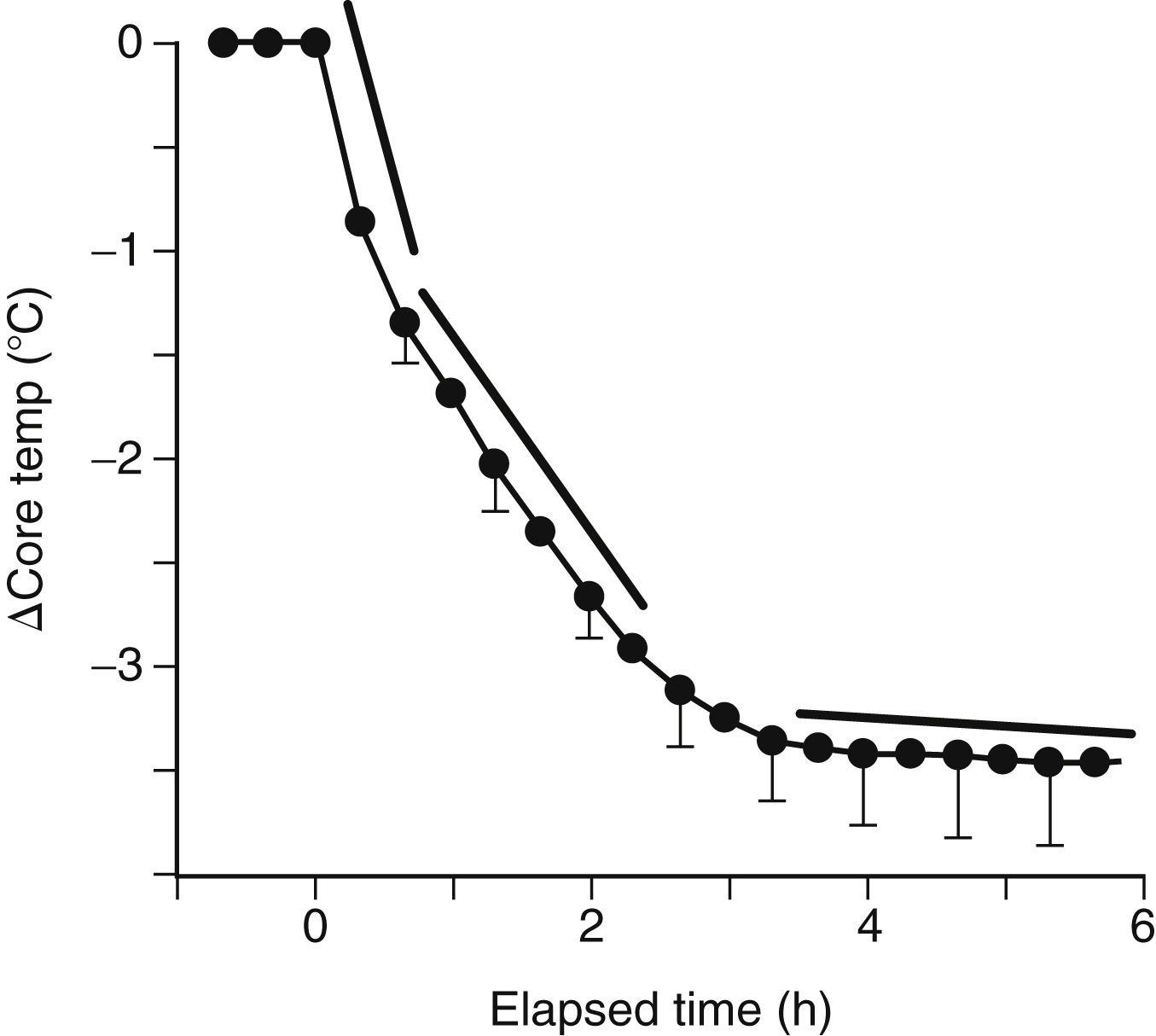
FIG. 53.1 Heat loss mechanisms of radiation, convection, conduction, and evaporation in patients under anesthesia. (From Nagelhout JJ, Plaus KL: Nurse anesthesia, ed 5, St. Louis, MO, 2014, Elsevier.)
Conduction
Conduction involves the transfer of heat energy through direct contact between objects. Conduction loss accounts for as much as 10% of heat loss in the OR and may occur via several mechanisms including patient contact with a cold OR table, skin preparation solutions, intravenous (IV) fluids, irrigants, and cold sheets and drapes.1,3,4
Convection
Convection involves the loss of body heat via transfer to the surrounding cooler air and occurs with a temperature gradient between the body and surrounding air. It accounts for 25% to 50% of heat loss in the OR. This transfer may occur in two ways. Passive movement occurs as a loss of body heat from basic skin exposure as warm air rises. Active movement, which can be facilitated by the laminar flow systems in an OR, occurs as a loss of body heat from a fan or wind blowing across the body surface.1,3,4
Evaporation
Temperature Measurement
Patients have rapid core temperature changes during the perioperative period. During such periods of rapid temperature fluctuation, a core temperature measurement provides the most accurate indication of body temperature. Temperature measurement during this period must be accurate and consistent and provide a true reflection of the core temperature measurement. The relationship between temperatures measured at various body sites during this period, however, may differ significantly from a true core reading. Consideration of the best method for obtaining a temperature must also take into account accessibility of the measurement site, patient comfort and safety, and the practitioner’s ability to consistently use the device correctly.2,4,6 The same route of temperature measurement should be used throughout the perianesthesia period to allow for accurate temperature comparisons across the surgical continuum, and any extreme temperature (hypothermic or hyperthermic) taken with a noncore measurement instrument should be interpreted with caution.2
The most accurate core temperature measurement is obtained via use of a pulmonary artery (PA) catheter because the artery bathes the catheter with blood from the core compartment and its surroundings. Temperature readings at the site can be affected by the rapid infusion of large amounts of warmed or cold IV fluids, by respiratory cycles, and by lower limb pneumatic compression devices. Readings from the distal esophagus and nasopharynx provide accurate alternatives to the PA catheter and are commonly used during surgery; however, like the PA catheter, these methods are invasive in nature and are not appropriate outside of the operative setting once the patient has been extubated.1,4,6
Oral temperature measurement with electronic digital thermometers is a popular method of temperature measurement that is easily accessible, less prone to operator error, and quickly reflects changes in core body temperature. Oral temperature readings vary based on placement in the oral cavity (Fig. 53.2).7 Oral temperature measurement taken in the right or left posterior sublingual (buccal) pocket provides an accurate reflection of core temperature even in the presence of oxygen therapy, warmed and cooled inspired gases, and varied respiratory rates.1,2,6,8
Temporal artery thermometry is a noninvasive radiation thermometer with use of a scanner probe to scan the forehead and capture the infrared heat from the arterial blood supply and lock in the highest temperature sensed. Current evidence supports the accuracy of temporal artery readings as a core temperature measurement at normothermic temperatures; however, the accuracy of temporal artery measures at temperature extremes has not been established.2,9–12
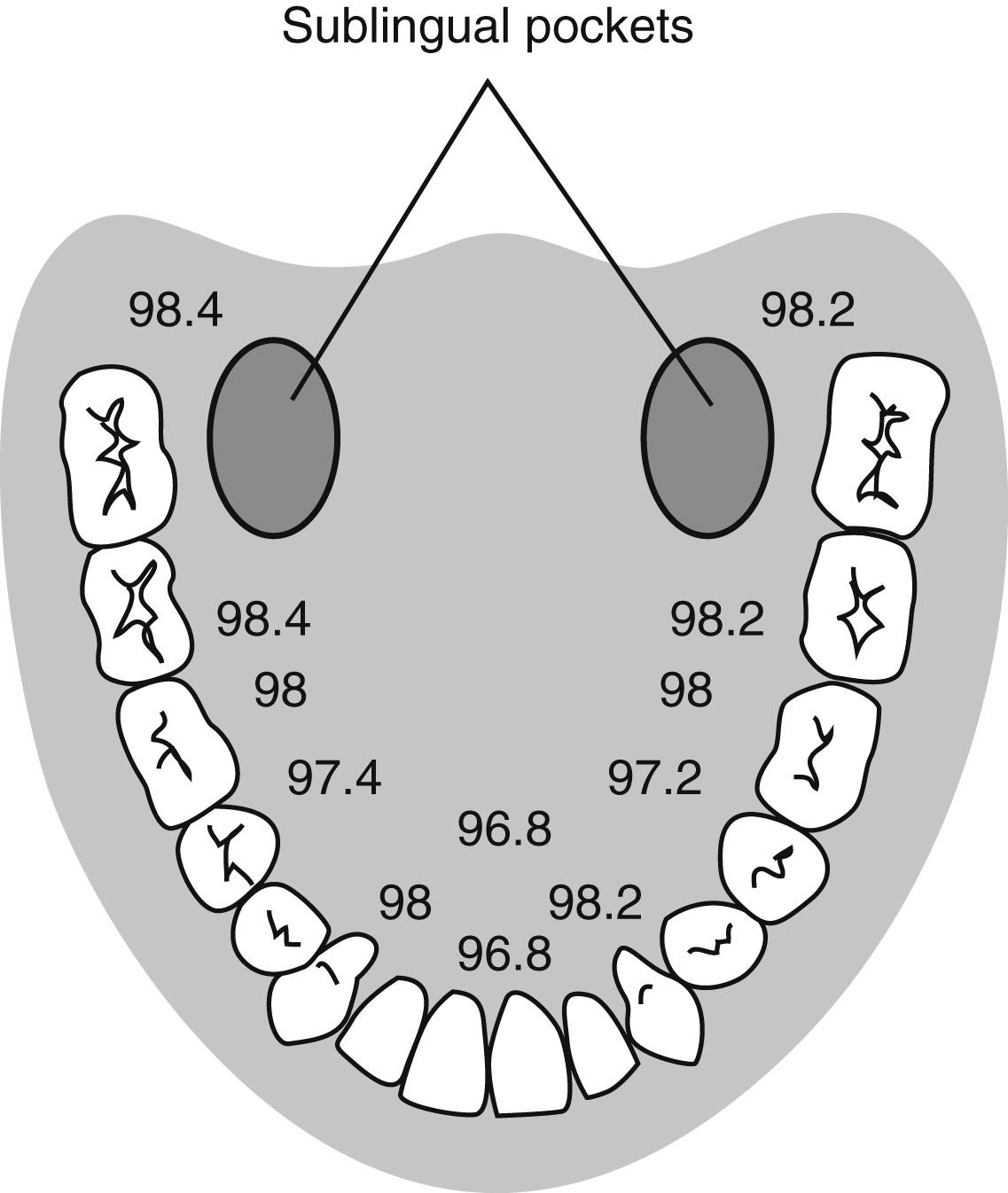
FIG. 53.2 Temperature variations in oral cavity. (From Nicoll LH: Heat in motion: evaluating and managing temperature. Nursing 32:s12, 2002.)
Infrared tympanic thermometry, previously considered a preferred method for noninvasive core temperature measurement, can be adversely affected by common sources of instrument error including poor operator technique, patient anatomy (site), and the calibration, accuracy, and inherent instrument error of the thermometer used.6 Given the issues surrounding temperature measurement with this instrument, it is no longer recommended as an accurate means of temperature measurement during the perianesthesia period.2
Perioperative Hypothermia
Perioperative hypothermia is defined as a core body temperature lower than 36° C.2,13 As many as 70% of surgical patients have hypothermia in the course of the surgical experience. All patients undergoing general and/or regional anesthesia are at risk for developing unplanned perioperative hypothermia; additional risk factors include2:
• Extremes of ages
• Female gender
• Systolic blood pressure less than 140 mm Hg
• Level of spinal blockade
• Length and type of surgical procedure
• Normal or lower than normal BMI
• Body surface/wound area uncovered
• History of diabetes with autonomic dysfunction
• Use of cold irrigants
• Use of general or regional anesthesia
Adverse effects associated with perioperative hypothermia include2:
• Patient discomfort
• Increased adrenergic stimulation
• Untoward cardiac events
• Coagulopathy
• Altered drug metabolism
• Impaired wound healing
• Surgical site infection
• Increased PACU and hospital length of stay
• Increased hospital costs
The typical temperature drop associated with perioperative hypothermia is between 1° C and 3° C and depends on the type and dose of anesthesia, amount of surgical exposure, and ambient room temperature. This temperature drop occurs from a loss of normal physiologic thermoregulatory mechanisms impaired by anesthetic drugs. As a result, the patient becomes poikilothermic and, without intervention, takes on the cooler temperature of the operative environment.3,14
Intraoperative temperature loss typically occurs in a characteristic pattern as a result of core-to-peripheral redistribution (Fig. 53.3). Redistribution occurs as a result of a reduction in the vasoconstriction threshold from the inhibitory effect of general anesthesia, resulting in a drop in core temperature and peripheral vasodilation triggered by both general and regional anesthesia, which causes an increase in the blood flow to the skin and a resulting loss in core body heat. An initial heat loss of 1° C to 1.5° C occurs during the first hour of surgery followed by a slower more linear drop over the next 2 to 3 hours. Core temperature loss generally does not stabilize until 2 to 4 hours into the surgical procedure (Fig. 53.4). Postoperative return to normothermia occurs once the brain anesthetic concentration decreases enough to allow a normal thermoregulatory response. This response may take as long as 2 to 5 hours to kick in and may be inhibited by residual anesthetics and postoperative opioids.3,4,14
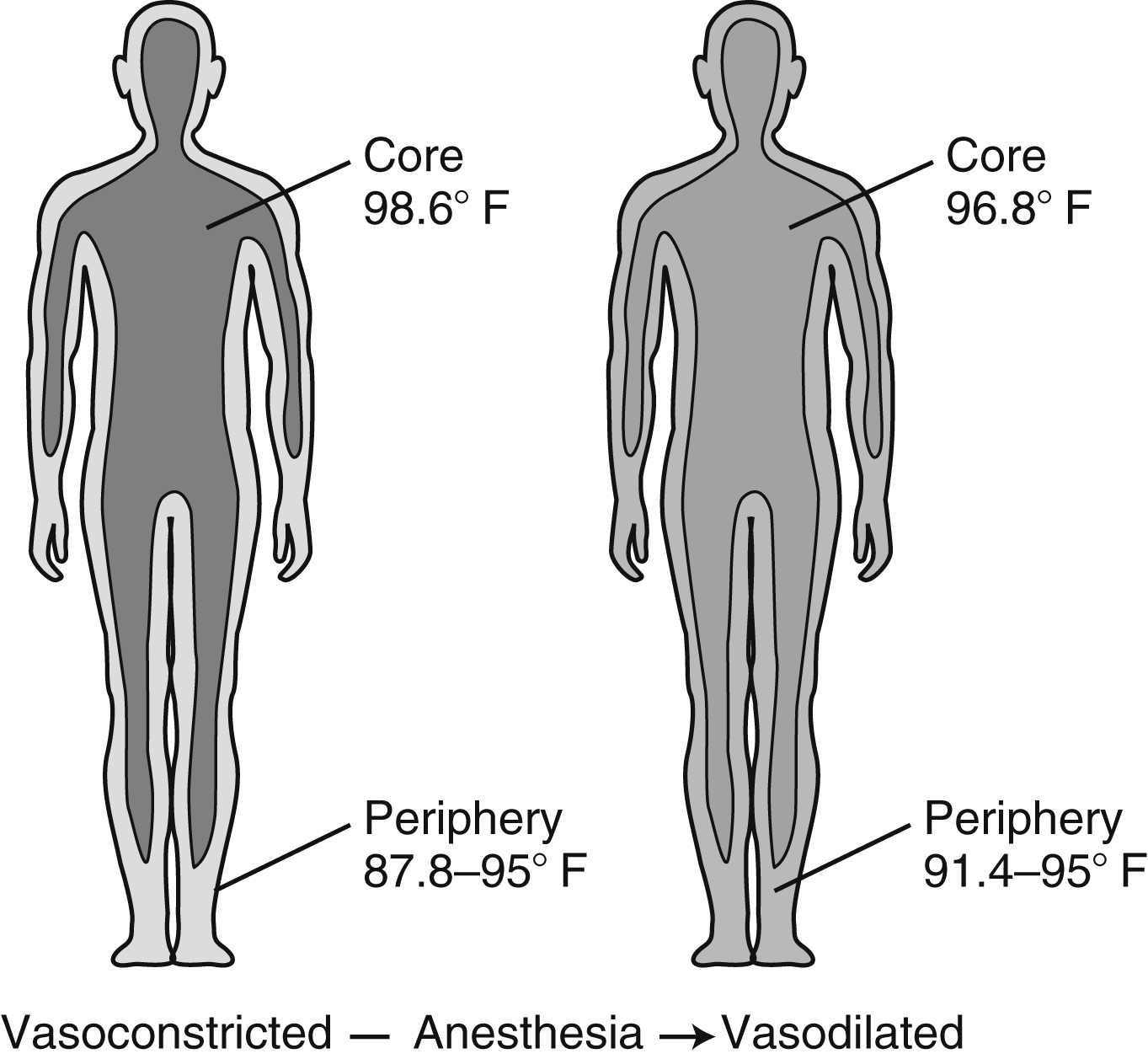
FIG. 53.3 Core-to-peripheral redistribution after administration of anesthesia. (From Sessler DI: Perioperative heat balance. Anesthesiology 92:583, 2000.)
Every patient should be assessed for hypothermia on arrival in the PACU, and postoperative care should be provided as per the multidisciplinary American Society of PeriAnesthesia Nurses’ (ASPAN) Clinical Guideline for the Maintenance of Perioperative Normothermia2 (Fig. 53.5). In the case of normothermia, preventative warming measures and passive insulation should be instituted. The ambient room temperature should be maintained at or above 24° C, and the patient’s thermal comfort level should be assessed at a minimum of admission and discharge. In addition to constant observation for the signs and symptoms of hypothermia, the patient’s temperature should be reassessed when the thermal comfort level decreases, with any emerging signs or symptoms of hypothermia, and on discharge from the PACU. In addition to the previous measures, active warming should be initiated for any patient with hypothermia who is admitted. In addition, IV fluids should be warmed, all gases (oxygen) humidified and warmed, and temperature monitored at least every 15 minutes until normothermia is achieved. The expected outcomes for all patients in Phase I PACU include a return to normothermia (minimum discharge temperature of 36.0° C), resolution of the signs and symptoms of hypothermia, and patient verbalization of an acceptable level of warmth. Preventative warming measures and continued assessment for hypothermia should continue at whatever location to which the patient is discharged.2
Malignant Hyperthermia
MH is a genetic abnormality of muscle metabolism initiated by certain triggering agents resulting in a hypermetabolic state. MH is precipitated by certain general inhalation anesthetics, depolarizing skeletal muscle relaxants, and stress.1,4,15–17 The incidence rate of MH ranges from 1 in 5000 to 1 in 100,000 anesthetics. MH averages about 600 cases per year in the United States, with hot spots including Wisconsin, Michigan, and West Virginia.17,18 A study of the clinical manifestations of MH in North America from 1987 to 2006 noted that almost 75% of MH presented in males and almost 70% of those incidences in Whites.19 The study showed that the most commonly presenting clinical symptom was hypercarbia followed by sinus tachycardia and masseter spasm19; additional signs and symptoms include generalized muscle rigidity, unstable blood pressure, tachypnea, mixed respiratory and metabolic acidosis, myoglobinuria, hyperkalemia, and fever that may exceed 110° F (43° C).1,4,15,16,19,20 Research shows that time from induction to first sign of MH was less than 30 minutes in the majority of cases.21 Once the acute episode is treated in the OR, the patient may be admitted to the PACU. Because successful management of MH depends on early assessment and prompt intervention, the perianesthesia nurse must be knowledgeable in the pathophysiology and treatment of this syndrome. Resources include the Malignant Hyperthermia Association of the United States (MHAUS) (available at www.mhaus.org) and North America MH Registry of MHAUS (available at www.mhreg.org).
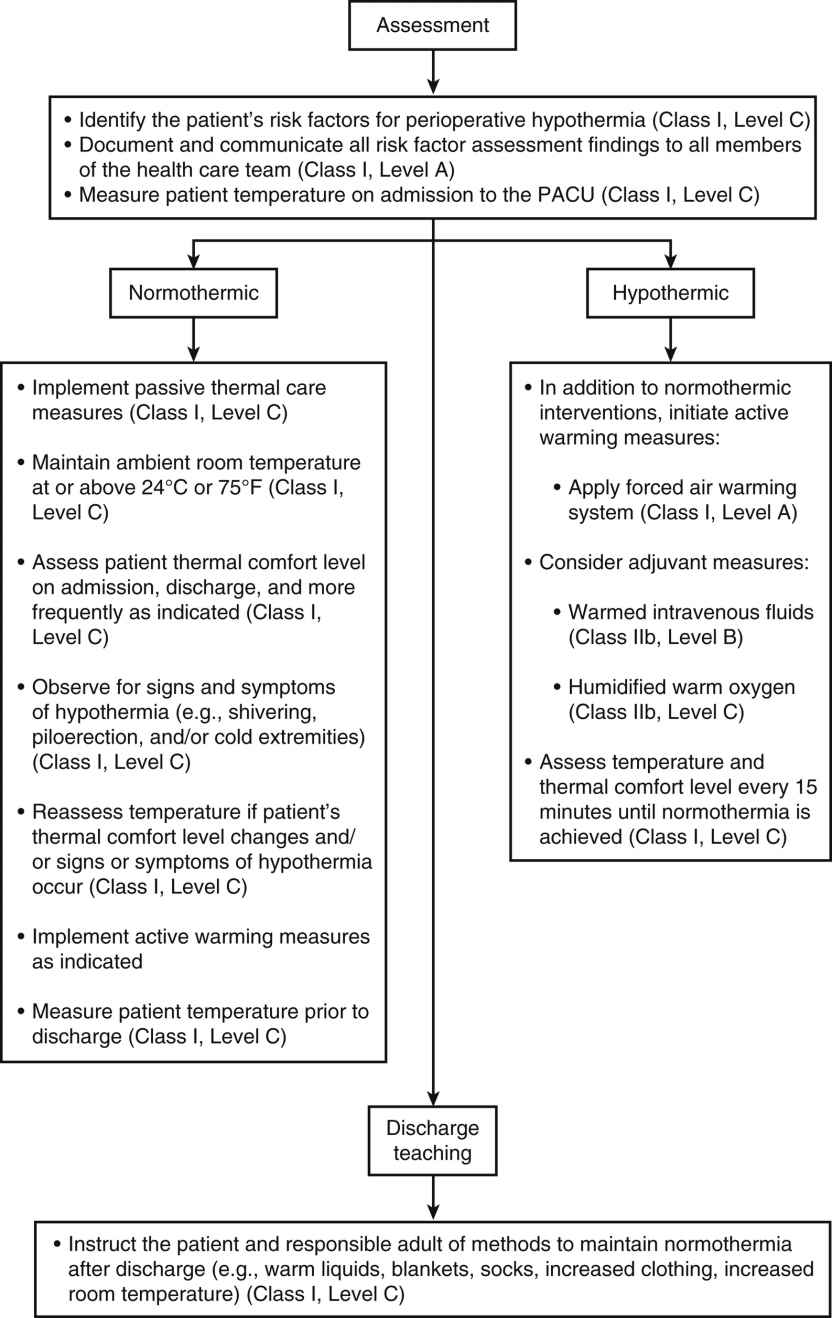
FIG. 53.5 Phase I/II PACU postoperative patient management recommendations. (From Hooper VD, Chard R, Clifford T, et al.: ASPAN’s evidence-based clinical practice guideline for the promotion of perioperative normothermia: second edition. J Perianesth Nurs 25(6):346–365, 2010.)
Identification of Patients With Malignant Hyperthermia Susceptibility
Genetics
Humans probably inherit susceptibility to MH with more than one gene or more than one group of possible mutational forms of a gene. The pattern of inheritance may range from recessive to dominant with graded variations in between. The ease of initiation of an episode of MH seems to depend on the degree of genetic susceptibility and on environmental factors, which explains why some patients who are susceptible show no signs of MH when exposed to confirmed MH-triggering agents on initial exposure but may react with later repetitive exposures. A patient with MH susceptibility (MHS) could be given an anesthetic in the presence of trigger agents, and this patient might not experience an acute MH reaction during surgery but could have MH develop in the PACU instead.1,15
Evaluation of Susceptibility
Before anesthesia is administered, identification of patients who may be susceptible to MH is of major therapeutic importance. On history and physical examination, patients with MHS usually show some subclinical muscle weakness or abnormality such as deficient fine motor control. Many patients with MHS have muscle cramps that occur spontaneously, during an infectious illness, or during or after exercise. When these cramps are present, they may be so severe that they are almost incapacitating. The patient may also describe heat prostration during physical exertion associated with environmental heat stress. In addition, a positive patient history or a positive genealogy may go back two generations (i.e., the patient or immediate relatives may show MH symptoms during an anesthetic experience). Physical examination of the person with MHS may reveal myopathies such as wasting of the distal ends of the vastus muscles and hypertrophy of the proximal femoral muscles of the thigh. Other myopathies associated with MH susceptibility are cryptorchidism, pectus carinatum, kyphosis, lordosis, ptosis, and hypoplastic mandible. Electromyographic changes are seen in fewer than half of patients with MHS. Electrocardiographic results of patients with MHS may reveal ventricular or atrial hypertrophy (or both), bundle branch block, myocardial ischemia, and ventricular dysrhythmias. Measurements of blood creatine phosphokinase (CPK) are usually about 70% reliable in estimating susceptibility to MH. The most definitive test for detection of MH susceptibility is the biopsy of skeletal muscle. Samples are obtained from the quadriceps muscle and are subjected to isometric contracture testing. The skeletal muscle of the patient with MHS has an increased isometric tension when exposed to caffeine or halothane.1,15,16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree