Key Clinical Questions
What are the indications for cardioversion?
What is the difference between cardioversion and defibrillation?
What is the difference between monophasic and biphasic cardioverter-defibrillators?
How much energy should be used during cardioversion?
What are the indications for anticoagulation prior to cardioversion?
Introduction
Cardioversion is a medical procedure whereby an abnormal heart rhythm (ie, cardiac arrhythmia) is converted back to a normal rhythm using electricity or drugs. Electrical cardioversion, also referred to as direct-current or DC cardioversion, involves the delivery of a synchronized (perfectly timed) electrical shock through the chest wall to the heart to terminate arrhythmias and restore sinus rhythm. Electrical cardioversion is an effective, rapid, and safe technique that has become a routine procedure in the management of patients with cardiac arrhythmias. Pharmacologic cardioversion, also called chemical cardioversion, uses antiarrhythmic medication instead of an electrical shock to restore the heart’s normal rhythm.
Investigators at Johns Hopkins Hospital were the first to develop techniques of defibrillation by an electrical shock in the 1930s. The first human defibrillation was performed in the operating room by Claude Beck in 1947, and Paul Zoll introduced defibrillation using alternating current in 1956. Direct-current defibrillation was subsequently pioneered and introduced into clinical practice by Bernard Lown in 1962. Subsequent studies in the early 1960s demonstrated that electrical cardioversion across the closed chest could abolish other cardiac arrhythmias in addition to ventricular fibrillation (VF). Today, atrial fibrillation (AF) is the most frequent arrhythmia encountered in clinical practice and the most commonly cardioverted arrhythmia.
Electrical cardioversion and defibrillation procedures both use a device (eg, cardioverter-defibrillator) to deliver an electrical shock to the heart to treat abnormal heart rhythms, but they differ in the timing of the shock and energy provided. Electrical cardioversion delivers energy synchronized (ie, perfectly timed) to the QRS complex and is used to treat arrhythmias such as AF, atrial flutter (AFL), or ventricular tachycardia (VT) with a pulse. Cardioversion can be performed electively (ie, nonemergently) in stable patients or emergently in situations to correct a rapid abnormal rhythm associated with faintness, low blood pressure, chest pain, difficulty breathing, or loss of consciousness. Cardioversion is not performed in pulseless patients.
Defibrillation delivers nonsynchronized (ie, occurs at random timing) energy during the cardiac cycle and is used to treat life-threatening arrhythmias such as pulseless VT or VF. Defibrillation is the treatment of choice for the arrhythmias most commonly associated with sudden cardiac arrest. The electrical shock delivered during cardioversion or defibrillation can be delivered externally to the heart using electrodes (external pads or paddles) placed on the chest (most common); directly to the heart using internal paddles during an open chest surgery; or through the electrodes of a permanently implanted cardioverter-defibrillator (ICD).
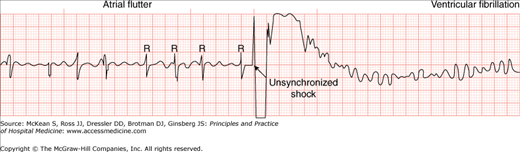
Cardioversion disrupts the abnormal electrical circuit(s) in the heart and restores a normal heartbeat. The shock causes all the heart cells to depolarize simultaneously, thereby interrupting and terminating the abnormal electrical rhythm without damaging the heart. This split second interruption of the abnormal beat allows the heart’s electrical system to regain control and restore a normal heartbeat. The delivery of energy during the shock is synchronized to the QRS complex, which represents cardiac depolarization, in order to terminate the arrhythmia safely and allow sinus rhythm to resume. The accidental delivery of an electrical shock to the T wave, which represents cardiac repolarization, is unsafe and may result in life-threatening ventricular arrhythmias (Figure 125-1). For this reason, synchronized cardioversion is used, rather than nonsynchronized defibrillation, for the abatement of abnormal rhythms with pulse.
Figure 125-1
A complication of cardioversion: induction of ventricular fibrillation. The ventricular arrhythmia occurred because the operator failed to enable the synchronizer, resulting in an inadvertent delivery of the shock on the vulnerable T wave instead of the intended delivery on the R wave. This complication is preventable by enabling the synchronizer and checking that it is properly functioning before shock delivery. (From Kerber RE. Transchest cardioversion: optimal techniques. In: Tacker WA, ed. Defibrillation of the Heart: ICDs, AEDs and Manual. St. Louis: Mosby-Year Book, 1994:164. With permission.)
|
Electrical cardioversion appears most effective in terminating arrhythmias that arise from a single reentrant circuit such as AFL, atrioventricular nodal reentrant tachycardia (AVNRT), atrioventricular reentrant tachycardia (AVRT), and monomorphic VT. The high-energy shock depolarizes the myocardium and the conduction tissue involved in the reentry circuit of the arrhythmia simultaneously. The depolarization of the myocardium is followed by a period of refractoriness that has the effect of interrupting the reentrant circuit. This interruption breaks the repeating cycle and terminates the arrhythmia. When the reentrant circuit is broken and the arrhythmia stops, the sinus node begins to fire again and a normal heart rhythm is restored. This mechanism of action does not explain how electrical cardioversion terminates AF, which arises from multiple reentrant circuits. As a result of the multiple circuits, successful cardioversion of AF often requires higher energy for termination. The exact mechanism by which electrical cardioversion terminates AF remains unknown. Other arrhythmias, such as junctional tachycardia and multifocal atrial tachycardia, originate from ectopic sites (ie, nonpacemaker) in the heart. Cardioversion is not effective for these types of arrhythmias.
Cardioverter-defibrillators deliver energy in a variety of waveforms, broadly characterized as monophasic or biphasic. Monophasic devices provide a unidirectional pulse of energy, meaning that electrons flow in a single direction through the heart. Monophasic energy is highly effective, and monophasic cardioverter-defibrillators remain widely used. Alternatively, biphasic devices deliver a sinusoidal biphasic waveform whereby the initial phase of energy is positive followed by a phase of opposite polarity, which means that during the shock the polarity and electron flow reverse. Biphasic cardioverter-defibrillators deliver a more consistent magnitude of current, which terminates arrhythmias more effectively and at lower energies compared to monophasic devices. As a result, biphasic cardioverter-defibrillators are increasing in use and have now become the standard for trans-chest defibrillation. Clinicians must distinguish which type of cardioverter-defibrillator is available for use, as this will dictate how much energy is required for successful cardioversion.
|
Indications and Contraindications
Electrical cardioversion has become a routine procedure in the management of cardiac arrhythmias. Indications include patients in whom the rate of the arrhythmia (eg, AF) cannot be adequately controlled medically or patients who poorly tolerate the arrhythmia (eg, palpitations, angina pectoris, and heart failure). Multiple cardiac rhythms may bear indications for cardioversion or defibrillation (Tables 125-1 and 125-2). The most common reason for the elective cardioversion of atrial arrhythmias is to enhance cardiac performance by restoring sinus rhythm, particularly in patients with mitral stenosis, left ventricular hypertrophy (aortic stenosis, hypertrophic obstructive cardiomyopathy), and diminished myocardial reserve (heart failure, myocardial ischemia, and infarction). Urgent cardioversion may be required for patients with atrial or ventricular arrhythmias who have myocardial ischemia, acute heart failure (pulmonary edema), hypotension, mental status changes, or worsening angina pectoris.
| Indications | Contraindications |
|---|---|
AF/AFL
Atrioventricular nodal reentry tachycardia Atrioventricular reentry tachycardia Ventricular tachycardia with a pulse |
|
Because electrical cardioversion is a low-risk procedure if properly performed, some authorities believe that every patient deserves one chance to achieve sinus rhythm by cardioversion, even if the short- or long-term prognosis of maintaining sinus rhythm is generally unfavorable. Other experts suggest that not all patients with newly discovered AF warrant an attempt at restoring sinus rhythm since several large, randomized trials have shown that the maintenance of sinus rhythm confers no survival (or other outcome) advantage over rate control. Treatment must be determined based on clinical parameters and patient preferences.
Contraindications to electrical cardioversion include rhythms that do not respond to electrical cardioversion (eg sinus rhythms, multifocal atrial tachycardia), digitalis toxicity, severe electrolyte imbalance (eg, hypokalemia), and known or suspected atrial thrombus. In AF or AFL, suspicion of atrial thrombus should be high if the rhythm onset is unknown or not clearly within the prior 48 hours. Transesophageal echo (TEE) evidence that the left atrium is free of thrombus may permit cardioversion for AF or AFL with unknown or > 48-hour onset.
Triage/Hospital Admission
Cardioversion is routinely performed in most hospitals in a closely monitored setting such as an intensive care unit, an emergency department, or a specially equipped procedure room. Although patients with atrial arrhythmias are often admitted to the hospital electively for cardioversion, outpatient cardioversion is a low-risk, effective, and economical procedure. If performed on an outpatient basis, patients are observed until most of the effects of sedation have abated (1–2 hours) and require transport home safely after the procedure due to the residual effects of anesthesia. Overnight hospitalization is seldom required.
Treatment
|
Habitus
Head and neck
Mouth
Jaw
|
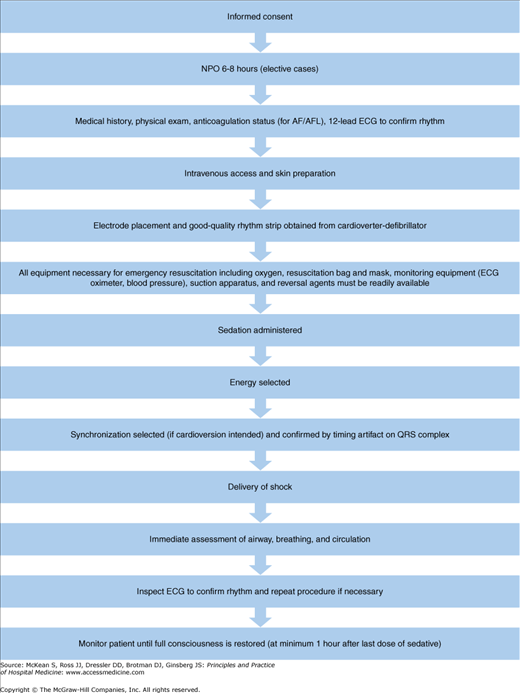
Prior to performing electrical cardioversion, structured measures should be integrated for all patients (Figure 125-2) as follows:
Informed consent (patient or decision maker)
Fasting (nothing oral) 6–8 hours prior to cardioversion (if elective)
History and physical examination
History should include current medications, allergies, previous adverse drug reactions or contraindications to sedation, diseases/disorders, prior hospitalizations, pregnancy status, nothing oral status, and anticoagulation status (if presenting arrhythmia is AF/AFL).
Use history to estimate duration of AF/AFL duration and determine need for anticoagulation and/or TEE evaluation.
Physical examination should include vital signs, oxygen saturation, pulmonary and cardiovascular examination, and oral cavity and airway examination. Patients who have an abnormal airway exam should be considered to be at increased risk for airway obstruction during sedation and may potentially have a difficult airway to manage if mask ventilation or intubation becomes necessary. Patients who present with any high-risk features (Table 125-3) should be considered for anesthesiology consultation prior to the initiation of the procedure.
A 12-lead electrocardiogram (ECG) is used to confirm rhythm.
If any electrolyte abnormality (hypokalemia, hyperkalemia) or drug toxicity (digitalis) is suspected, appropriate blood levels should be checked.
Peripheral venous access (for preprocedure sedation and postprocedure medications or fluids if needed) is obtained.
Skin preparation includes shaving of the chest hair, if present (with electric clippers rather than a blade) to ensure good contact of the skin and electrode pads. High-quality continuous ECG from the cardioverter-defibrillator will confirm proper contact of electrode pads to the skin.
Emergency resuscitation equipment available (Figure 125-2).
|
Cardioversion may be associated with pulmonary or systemic embolization. This complication is more likely to occur in patients with AF/AFL > 48 hours (ie, high-risk group) who have not been anticoagulated prior to cardioversion. In case-control series, the estimated risk of thromboembolism is between 1% and 5% in (nonanticoagulated patients) with AF/AFL > 48 hours undergoing cardioversion.
In patients with AF/AFL >48 hours, oral anticoagulation with warfarin (International Normalized Ratio (INR) 2.0 to 3.0) for 3–4 weeks prior to cardioversion reduces the thromboembolic risk to 0.5–0.8%. Anticoagulation before cardioversion allows time for adherence of any preexisting atrial thrombus and for prevention of new thrombus formation. Alternatively, anticoagulation with intravenous unfractionated heparin can be initiated and a TEE performed to evaluate the left atrium and left atrial appendage for evidence of thrombus. If there is no evidence of thrombus, cardioversion can safely be performed (thromboembolic risk 0.8%). The 2008 American College of Chest Physicians (ACCP) guidelines recommend that patients undergoing urgent cardioversion be heparinized as soon as possible to prevent thrombi from forming due to atrial appendage dysfunction after cardioversion. Cardioversion in patients who present with acute AF/AFL < 48 hours (ie, low-risk group) is associated with a low clinical rate of thrombembolism (0.8%).
|
Late thromboembolic events after cardioversion are probably due to both the development of thrombus as a consequence of atrial stunning and the delayed recovery of atrial contraction after cardioversion. Pooled data from 32 studies of cardioversioon of AF/AFL suggest that 98% of clinical thromboembolic events occur within 10 days of cardioversion. Anticoagulation after the procedure prevents thrombus formation from occurring before normal atrial mechanical activity has resumed, a process that may be delayed for several weeks due to atrial stunning. The American College of Chest Physicians (ACCP) and American College of Cardiology/American Heart Association, and the European Society of Cardiology (ACC/AHA/ESC) guidelines therefore recommend continuation of warfarin therapy for at least 4 weeks after cardioversion. This recommendation only addresses protection from embolic events related to the cardioversion period. Patients with AF at high risk from thromboembolic events based on CHADS2 score (an acronym for Congestive heart failure, Hypertension, Age > 75, Diabetes mellitus and prior Stroke or transient ischemic attack) may require long-term anticoagulation (see Chapter 126).
Care standards require administration of anticoagulant medications when preparing patients with AF/AFL of > 48 hours for cardioversion. The ACC/AHA/ESC guidelines for the management of anticoagulation in patients with AF/AFL undergoing cardioversion recommend anticoagulation for at least 3 weeks prior and 4 weeks following cardioversion if the abnormal rhythm has been present for > 48 hours, with exceptions based on TEE evaluation (Table 125-4).
Class I—There is evidence and/or general agreement that the following approaches are effective for the prevention of thromboembolism in patients with AF undergoing cardioversion
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|