Fig. 16.1
The increase in the absolute number of percutaneous coronary interventions versus the number of open heart surgeries from 1983–1985 to present. X axis: year. Y axis: number of interventons. According to the 2013 report of the “Nederlandse Hartstichting” (Dutch Cardiac Foundation). Black lines depict absolute number of annual cardiosurgical intervention (open hart operaties) as compared to the number of percutaneous coronary interventions (PCI) represented by the red line
In 2012, 17,293 open heart operations were performed on a total population of 16.7 million, which represents 104 open heart operations per 100,000 inhabitants [3]. Fifty-five percent of these were isolated coronary artery bypass grafting (CABG) and 42 % were isolated valve surgery. The remainder of the cases were for combined valve with CABG surgery, or other less prevalent forms like repair of the thoracic aorta, heart transplants, and congenital cardiac surgery [3]. Most valve surgery is for degenerative valve disease, and the proportion of valve operations due to delayed consequences of rheumatic fever is scant and mainly among patients born in other countries with a higher incidence of rheumatic fever in childhood. In the Netherlands, only 761 of the 17,293 cases of cardiac surgery were performed for children younger than 14 years.
Table 16.1
Adapted from (Yusuf et al. [2]). Mortality from Cardiovascular Disease and Infectious Causes in 1990, by region and gender (in thousands)
Region | Cardiovascular causes in men | Infectious causes in men | Cardiovascular causes in women | Infectious causes in women |
|---|---|---|---|---|
India | 611 | 429 | 481 | 240 |
China | 576 | 158 | 439 | 89 |
Other Asian and Pacific Island | 289 | 147 | 226 | 140 |
Commonwealth of Independent States | 416 | 20 | 253 | 6 |
Sub-Saharan Africa | 183 | 215 | 211 | 228 |
Latin America/Caribbean | 186 | 62 | 147 | 48 |
Middle East Crescent | 258 | 56 | 215 | 35 |
Established Market Economies | 483 | 42 | 227 | 12 |
Total | 3,028 | 1,128 | 2,201 | 798 |
Lowest Income Countries
On the other end of the spectrum are the poorest countries that have few local, national, or private sector resources and are categorized by the World Bank as Low Income Countries (LICs). Sixty percent of the burden of cardiovascular disease is borne by LICs, and the epidemiology of cardiac surgical disease is different in these countries [4].
According to the World Heart Federation (WHF), the burden of rheumatic heart disease—which is a consequence of an untreated or undertreated streptococcal upper respiratory infection—falls disproportionately on the children and young adults in LICs. In LICs alone, 15.6 million people are affected by rheumatic heart disease, two million per year require hospitalization, and 233,000 deaths per year are attributable to end-stage rheumatic heart disease. In some parts of sub-Saharan Africa, Latin America, and Asia and among the indigenous people of Australia and New Zealand, as much as 1 % of school age children have symptomatic rheumatic heart disease [5]. Though few international resources are dedicated to it, an estimated 16–18 million people in LICs are infected with Chagas disease, a parasite carried by a beetle that lives in mud and dirt structures that eventually causes an infectious cardiomyopathy [6]. Though infectious etiologies of surgical cardiac disease are not likely to be eradicated in LICs in the near future, these countries are beginning to confront ischemic heart disease due to the obesity epidemic and ischemic heart disease is expected to grow more quickly in LMICs than in HICs [6, 7]. According to the World Health Organization (WHO), in 2011 “Coronary disease killed more than 7 million people and stroke killed nearly 6 million. Most of the deaths were in developing countries” [4]. Thus, the burden of surgical cardiac disease will continue to fall disproportionately on the people with the least access to medical care and surgical services.
This author (PR) can easily recall three cases from Médecins Sans Frontières (MSF) emergency response humanitarian projects:
In Somalia, a pregnant woman presented at the emergency department in overt cardiac failure clinically diagnosed as aortic insufficiency.
In Liberia, a middle aged male visitor in the hospital suffered sudden cardiac death, presumably from a myocardial infarction.
In Somalia again, a newborn infant remained cyanotic after birth, a condition that did not improve with oxygen by face mask and a clinical diagnosis was made of cyanotic heart disease of unspecified cause.
In these cases, as in many cases of cardiac disease encountered in LICs, “clinically”, “presumably” and “unspecified” are the key words. As this author did, visiting anesthesiologists and local practitioners alike find themselves feeling ignorant and impotent in such clinical situations—“ignorant” because the exact diagnosis cannot be known without ECG and echocardiography equipment and an appropriate laboratory services, and “impotent” because even if the correct diagnosis were revealed, resuscitation or curative operation was not possible because of a total absence of equipment and tools. The number of these cases that might be prevented by good primary health care or detected by an adequately functioning hospital and cardiology care is not documented.
These three cases are representative of the burden of cardiac surgery in the poorest countries. To have an impact, congenital heart disease, the treatment of rheumatic fever, and the causes of emerging degenerative cardiac disease such as aging, urbanization, and lifestyle changes (smoking, decreased physical activity, obesity, and nutrition must all be addressed from a public health perspective. Goal oriented resource management can only be achieved when adequate data regarding existing health conditions are provided for low- and middle-income countries [8].
Middle-Income Countries
BRICS Countries
In between these socioeconomic extremes, on the top of the bell curve, lie the middle-income countries. This group is too diverse to describe as a whole. On one hand there are the so-called BRICS—Brazil, the Russian Federation, India, China, and South Africa—the countries representing three billion habitants of our planet. This term was first coined in a white paper by a Goldman-Sachs executive in 2001 to describe the investment opportunities in these up-and-coming economies [9]. This group of countries holds a yearly international summit; Argentina, Indonesia, Turkey, Iran, Nigeria, Syria, and Egypt are sometimes practically grouped in the same category, but do not take part in the BRICS economic summit [10].
Though the level of health care is considerably better than in the developing countries, it is far below what would be expected in the G7 countries, the most important differences being a marked disparity in the quality of health care provided and even more important unequal access to health care [11]. The disparity in access occurs for a variety of reasons such as a lack of universal social security system as in India and China or a marked urban–rural quality of life disparity as in South Africa and Brazil. Lack of health insurance providers in countries like India and China is also a primary cause of unaffordability in treating cardiac surgical cases [12]. Still many hospitals and university centers in BRICS nations may provide advanced medical care including advanced cardiac surgery at a standard of care comparable to that offered in so-called developed nations. The case mix is different from what may be seen in the developed countries with a substantial proportion of valve surgery due the late consequences of rheumatic fever and a much younger population of CABG patients owing to unhealthy lifestyle and “iceberg phenomenon” of diabetic patients [11].
BRICS countries have the personnel and expertise to improve the quality of their cardio-surgical care and the political potential to improve accessibility, but cooperation between practitioners and institutions in BRICS countries and HICs can still be fruitful. For host countries, these partnerships sharpen new techniques and improve academic rigor, and the visiting practitioners profit from the rewarding teaching and learning experience of helping a growing anesthesiology department or cardiac surgical program. Indeed the creative and innovative solutions employed by practitioners and health care systems in BRICS in order to invest limited resources effectively will be of interest to all health care practitioners. Health care professionals universally face the changing landscape created by the global economic crisis and can subsequently implement these measures in their home country.
Other Middle-Income Countries: The Target Group?
At the lower end of the MIC group, are the countries that have less immediate economic potential or are lagging behind in development as compared to these BRICS countries. Very probably realpolitik will drive the majority of cardiac surgical missions to these countries where primary and secondary care is sufficiently developed to be able to consider doing cardiac surgery (Fig. 16.2).

Fig. 16.2
Peeping through the window of the cardiac OR in Suriname. Suriname is considered an upper middle income country by the World Bank, which means the Academisch Ziekenhuis of Paramaribo, the academic hospital, has sufficient resources to support a cardiac surgical mission
Congenital Cardiac Surgery
Discussing cardiac surgical disease is incomplete without mentioning congenital cardiac heart disease. Structural heart disease affects infants and adults in high-, low-, and middle-income countries with relatively the same genetic prevalence—8–12 cases/1,000 live births, with the rate depending on the method of detection—but the burden of disease falls disproportionately on the lowest income countries [13–15]. This disparity exists not only because of lower per capita income to support expensive care but because higher fertility rates in LICs translate to more cases of congenital heart disease per wage earner [13]. Although only a small proportion of cardiac surgical care in any setting is provided to infants and children, the effect of treatment can provide 20–50 Disability adjusted life years (DALYs)3 per case treated in the population in question [16]. Timely detection followed by curative surgical treatment may normalize life expectancies as well as improve neurodevelopmental outcomes for a considerable proportion of congenital cardiac malformations as well as cardiac disease due to infectious causes [15, 17]. The problem of congenital heart disease must be addressed from several angles with increased attention for mother and child survival campaigns, improved capacity of medical and diagnostic services, and finally improved access to cardiac surgical services [6]. As we discuss below, for successful treatment of any cardiac surgical disease—including congenital heart disease—several layers of care and capability must be in place to detect congenital cardiac malformations in an early stage, provide timely treatment, and to propose appropriate follow-up programs.
Summary
Cardiovascular disease has a huge impact on the global burden of disease, an impact that will continue to grow in particular among the populations of LMICs, who are not only affected by infectious cardiac disease, but have an increasing incidence of ischemic and degenerative disease. Although the largest impact in many countries will come from a focus on primary prevention, cardiac surgical care is an indisputable part of public health concern. The particular burden of disease and capacity to build surgical services will be different in every country and how that should be organized will very much depend on the specific context of the country. Typically a minimal level of primary and secondary health care will be necessary in order to be able to deploy a cardiac surgical development program. For more comprehensive review the reader is referred to an excellent article of Pezzella et al. [18] In the next section, we discuss the task of assessing capacity, forming partnerships, and making goals for a cardiac surgical program.
Cardiac Surgical Mission Goals and Preparation
Goals and Modalities of a Cardiac Surgical Mission
When one is choosing to participate in a humanitarian surgical mission, it is a good idea to think about how one’s personal goals and the goals of the organization align. Especially for a cardiac surgical mission, the goals of the trip will dictate the daily activities and responsibilities, which may range from simply providing the anesthetic to helping plan the case schedule to providing postoperative ICU care. These can also include teaching a team of local practitioners how to expand their practice to implement the new procedures performed.
Defined goals of cardiac surgical missions may include:
Bringing patients with advanced disease from LMICs to a cardiac surgical center in HIC and repatriating the patient after recovery.
Performing cardiac surgery and anesthesia in a well-equipped but temporary structure, e.g., an empty wing of a hospital.
Performing cardiac surgery and anesthesia in a local facility in conjunction with local practitioners.
Assisting in setting up or planning a new cardiac surgical facility.
Providing education for local practitioners, residents, fellows, nurses, or perfusionists.
Participants should look for programs where the host institution has a voice in the design and implementation of the program. Reputable programs will also be recognized by the host country Ministry of Health (MoH) and host national professional associations. Often local or international nongovernmental organizations (NGOs) will play a role in the organization and logistical support. The technical expertise can come from international specialty associations (in anesthesia and surgery). Regardless of who the players on the ground are, looking for an organization that emphasizes sustainability and self-sufficiency of the host team and training of specialists as opposed to substitution of roles will ensure the best experience for the visiting practitioner, host institution, and ultimately the population of the host country.
The Premise of a Mission
Making Voluntarism Meaningful and Impactful
Many authors in this text have spoken out against medical tourism in its many forms. That argument will not be repeated here; however, it is worth noting that more than any other subspecialty care, the treatment and care of cardiac surgical diseases requires long-term care from a team of health care providers including: cardiologists, cardiac surgeons, cardiothoracic anesthesiologists, perfusionists, intensivists, operating room technicians, and last but certainly not least nurses with experience in the OR, ICU, post-anesthesia care unit (PACU), and clinic.
Dr. Aldo Castañeda4 noted in his address to the World Society for Pediatric and Congenital Heart Surgery (WSPCHS) in Antigua, Guatemala in 2010, “occasional visits of traveling teams performing a few diagnostic tests, interventions, and operations…will not solve the permanent needs of a cardiac unit in a developing country” [19].
Dr. Castañeda’s work is one of the best examples of holistic surgical service capacity building. Since 1997, the Fundación Aldo Castañeda has facilitated growth of the Paediatric Cardiac Surgical Unit of Guatemala, expanding the capacity for outpatient consultation, echocardiography diagnostics, preoperative, intraoperative, and postoperative care of cardiac surgical patients, and graduate medical education [20, 21]. As of 2012, the Paediatric Cardiac Surgical Unit of Guatemala had tripled the number of surgeries performed annually, had become an international referral center for pediatric cardiology, and had begun to train subspecialist physicians in pediatric cardiac anesthesiology, pediatric cardiology, pediatric critical care, and cardiopulmonary perfusion [20]. Whether it is through “developing a functioning, independent local effort” [19] as Dr. Castañeda has done in Guatemala and as Surgeons of Hope® has done in Nicaragua, through ongoing collaboration between university departments in HICs and LICs, or by a establishing a meaningful, long term education program, enhancing the ability of local practitioners to safely care for the needs of their community should be the ultimate goal.
The most explicit example of this philosophy is described by the organization Children’s HeartLink®, which was founded by Drs. Frank Johnson and Joseph Kaiser cardiovascular surgeons at Swedish Hospital in Minneapolis, Minnesota. In the early 1990’s, Children’s HeartLink® transitioned from providing care for children from LMICs in the USA to partnering with local hospitals and practitioners. Currently Children’s HeartLink® practices a well-delineated process of local surgical capacity building with its sites; during this 6- to 10-year process they call the “Phased Support Approach,” they work with sites on three continents to achieve a state where local practitioners are able not only to perform surgeries with acceptably low complication rates, but to train other practitioners [22, 23].
The transition from service substitution to independence often evolves organically without a well set plan, as in the example of the experience of one of the authors (PR) with the setup of a cardiac surgery program in Suriname. Until the late nineties Suriname cardiac patients were operated in The Netherlands, through a program which was financed by the Dutch Government. When this agreement was stopped for political reasons, one German and three Dutch cardiac surgical departments joined efforts with the Academic Hospital of Paramaribo to organize three to four 3-week missions per year. During these missions, an average of 35 patients were operated upon; these patients were pooled in advance by Suriname Cardiologists. The effort required to keep up this frequency of missions was challenging and required dedicated perseverance in particular from a single cardiac surgeon (GJ Kootstra) from Breda, The Netherlands, Gradually more coordinated efforts for collaboration and training of cardiac anesthesiologists and perfusionists were introduced involving three one-year fellowships in Amphia Hospital, supported by a grant from the European Association of Cardiothoracic Anaesthesiologists (EACTA). This additional training bolstered the ICU, pharmacy, and organization of the cardiac surgical mission (Fig. 16.3).
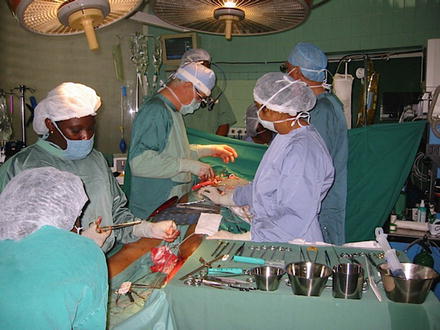

Fig. 16.3
A mixed surgical team at work on a coronary artery bypass graft (CABG). Local OR nurses are harvesting the venous graft while the visiting surgeons prepare the arterial target sites. This is an example of the middle stages of transition from a substitution team to a fully trained local team
Through 10 years of collaboration with Surinam Hospital management and cardiologists, what started a volunteer-based and externally financed project ended in a fully independent cardiac surgical department in Suriname fortuitously staffed by a Dutch cardiac surgeon eager for the opportunity to direct a fully functional cardiac surgical unit in his native country.
Partnering for Sustainability
Unless the organization’s mission is explicitly dedicated to cardiac surgery—as is the case with several organizations mentioned above—local nongovernmental organizations (NGOs) often have profound knowledge of the local context but lack specific expertise in cardiac surgery. The advantage of partnering with local NGOs is that the surgical team gains entrée into the community. In the experience of the author (PR), the disadvantage is that local NGOs will be driven by their funding streams, which are tied to achievement of short- and medium-term goals with high impact or great visibility. Partnering with local NGOs may mean an open time schedule if no interest exists in finalizing funded project.
Hospitals have access to all of the supply chains and structural implements for surgical missions, but administrators often lack knowledge of the larger national context as they are focused on maintaining private practices, serving the patients in need, and keeping the doors open. In working with any hospital, one also must deal with the microcosm of personalities and habits governing health care business as usual.
As successful university collaborations and NGOs such Children’s HeartLink®, Surgeons of Hope®, and Fundación Aldo Castañeda have demonstrated, the ideal situation may be the hybrid approach of connecting a host hospital where practitioners are ready to build a program with a functional team of practitioners with the support of an NGO or professional association. The Surinam example shows that this can be done without an NGO, but considerable effort will be need to overcome administrative and logistical hurdles.
With all of this in mind, physicians and nurses who are inspired to serve need not wait for the ideal situation to act or volunteer on a cardiac surgical mission—many anesthesiologists have had very fulfilling volunteer experiences in the absence of one or more of the conceptual elements described here [24–26]. No volunteer experience, even a surgical mission, comes with the guarantee of success and future productive academic relationships. Not all sites will become fully functioning independent adult or pediatric heart centers. But in order to help patients, one does need to know that minimum standards of quality and safety will be met. These standards are discussed in the next section.
Cardiac Anesthesia: Providing Optimal Care in Low Resource Settings
In 2008, the World Health Organization (WHO) published guidelines identifying multiple recommended worldwide safe surgical practices (see Table 16.2) [27]. In order to be able to practice any type of surgery in LMICs, these minimum conditions need to be fulfilled. In 2010, the World Federation of Societies of Anaesthesiologists (WFSA) updated the existing international standards for a safe practice in anesthesia and correlated these standards with the three levels of a hospital structure as defined by the WHO [28]. Although the WFSA guidelines were created for anesthesia for non-cardiac surgery, a cardiac anesthesia unit or visiting medical team should comply with all grades of suggestions and recommendations applicable for a level 3 hospital structure (see Table 16.3).
Table 16.2
WHO Objectives of Safe Surgery
Objective | |
|---|---|
1 | Operate on correct patient and site |
2 | Use anesthetic methods known to prevent harm and treat pain |
3 | Be prepared for airway emergency or respiratory failure |
4 | Avoid or appropriately treat adverse drug reactions |
5 | Recognize and treat large volume blood loss |
6 | Minimize risk of surgical site infections |
7 | Prevent retention of surgical instruments or implements |
8 | Label all specimens correctly |
9 | Communicate effectively for the exchange of critical information |
10 | Establish and participate in routine surveillance at the hospital system level |
Table 16.3
Standards for infrastructure, supplies, and anesthesia at the Referral Hospital Level
Level 3 (Should meet at least highly recommended, recommended and suggested anesthesia standards) Referral hospital |
|---|
A referral hospital of 300–1,000 or more beds with basic intensive care facilities. Treatment aims are the same as for Level 2, with the addition of: Ventilation in OR and ICU Prolonged endotracheal intubation Thoracic trauma care Hemodynamic and inotropic treatment Complex neurological and cardiac surgery Basic ICU patient management and monitoring for up to 1 week : all types of cases, but possibly with limited provision for: multi-organ system failure Hemodialysis Prolonged respiratory failure Metabolic care or monitoring |
Essential procedures Same as Level 2 with the following additions: Facial and intracranial surgery Bowel surgery Pediatric and neonatal surgery Thoracic surgery Major eye surgery Major gynecological surgery, e.g. vesico-vaginal repair |
Personnel Clinical officers and specialists in anesthesia and surgery |
Drugs Same as Level 2 with these additions: Propofol Nitrous oxide Various modern neuromuscular blocking agents Various modern inhalation anesthetics Various inotropic agents Various intravenous antiarrhythmic agents Nitroglycerine for infusion Calcium chloride 10 % 10 i.m. injection |
Donabedian described in 1988 how the quality of medical care could be assessed and assured in a three dimensional model [29]. He described three criteria that should be addressed to achieve an acceptable level of quality: structure, process and outcome criteria.5 In order to discuss specifically the minimum standards for cardiac anesthesia, the Donabedian model will be used.
Structure
The Regional Context
The socioeconomic environment will have some bearing on the type of cardiac surgical care that can be provided. Given the nature and the extent of the investment needed in cardiac surgery, local cardiologists must have infrastructure in place to be able to identify patients in sufficient numbers, prepare them for surgery, and support them after visiting practitioners leave. Often this will imply that a cardiac surgical mission is best planned in an urban area. In particular staff, equipment requirement and on-site logistical support will necessitate good geographic accessibility (good roads, proximity to airport).
The Hospital
Cardiac surgery and anesthesia can only be performed in a qualified hospital structure, preferably a referral hospital. As a cardiac surgical project or mission will create a spin-off effect that might benefit or draw on the resources other departments, affiliation with a university or teaching hospital should be actively sought out. It is perhaps out of touch with reality to say that the hospital should be well organized and functional—as there are so many hospitals in HICs that are not—but a thorough check on a secure water supply, electricity, medical gasses and waste management should be performed and confirmed to comply with minimal standards.6 An adequate number of qualified medical and support staff from the host site should be available around the clock. A reliable telecommunication system (within the hospital and outwards) needs to be in place (Fig. 16.4).

Fig. 16.4
Even with a thorough site evaluation, one must always be ready for the unexpected change in the infrastructure. Here, for example, the whole team transports a patient—stretcher and all—one floor downstairs from the OR to the ICU because the electricity and therefore the elevator had gone out
The hospital should offer most major categories of specialty care (at minimum cardiology, internal and pulmonary medicine, general surgery and paediatrics). Intensive care staffing and work patterns for monitoring postoperative patients may be considered adequate by the host team, but will not meet standards of care of the visiting team [26]. Specific inquiries should be made to determine if intensivists needs to be added to the visiting team. Specifically for cardiac surgery, the availability of transesophageal echocardiography (TEE) machines and probes and bronchoscopy equipment appropriately sized for the surgical population needs to be determined.
In an ideal situation, cardiology department should be fully functional with a qualified staff of interventional cardiologists, equipped with a cardiac catheterization laboratory and echocardiography equipment and expertise. A local cardiologist must be available for postoperative care and for emergency interventional procedures. Ideally, the hospital would have a dedicated ICU and cardiac surgical ward with 24/7 nursing staff. If this is not available, provisions must be made so all cardiac surgical patients can be hospitalized and monitored preoperatively and postoperatively. If a ward or a smaller hospital will be repurposed for PACU, ICU, and ward, this space should be equipped with ventilators, oxygen saturation and invasive blood pressure monitoring, a telemetry system, a central oxygen supply system, medical air and vacuum pipeline system for connecting suction drainage systems.
Laboratory and Imaging Services
A well-staffed laboratory that can perform chemistry, hematology, and hemostasis tests will be needed providing service around the clock. Substituting an accurate Point of Care (POC) laboratory unit for hemoglobin, glucose, blood gases with electrolytes, and/or dynamic hemostasis may prove to be effective and efficient. Radiology services need to be available on a daily basis for diagnostics [30–32].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






