Cardiorespiratory Interactions in Children With Heart Disease
Ronald A. Bronicki
Lara S. Shekerdemian
KEY POINTS
 In healthy individuals, spontaneous breathing and positive pressure ventilation (PPV) primarily affect the right heart.
In healthy individuals, spontaneous breathing and positive pressure ventilation (PPV) primarily affect the right heart. Ventilation has important effects on left ventricular afterload. A positive intrathoracic pressure can reduce afterload, and a negative intrathoracic pressure can increase it.
Ventilation has important effects on left ventricular afterload. A positive intrathoracic pressure can reduce afterload, and a negative intrathoracic pressure can increase it. PPV may be beneficial for children with systolic heart failure, and it can be delivered by the noninvasive route.
PPV may be beneficial for children with systolic heart failure, and it can be delivered by the noninvasive route.INTRODUCTION AND HISTORICAL PERSPECTIVE
The term cardiorespiratory interaction describes the physiologic relationship between spontaneous or positive pressure ventilation (PPV) and the cardiovascular system. These interactions can be exaggerated or abnormal in certain disease states. The complex interactions that are present in congenital heart disease have formed the basis of extensive research, and ventilation is now routinely used as a hemodynamic tool in many circumstances. This chapter will include a brief overview of the evolution of our understanding of cardiopulmonary physiology and a discussion of the interactions between ventilation and the cardiovascular system, with particular reference to critically ill infants and children with congenital and acquired heart disease.
The Foundation of Our Current Knowledge
The ancient Greeks called the air that we breathe the “breath of life.” Galen believed that the lungs served to provide the blood with heat from the inhaled air, which was then carried to the heart. He also described the functions of venous and arterial blood. The venous blood was responsible for nutrition, and arterial blood delivered vitality from the heart to the body’s organs. The Galenic circulation did not recognize the pump function of the heart and the pulsatile nature of the arteries, or the passive pressure gradient for venous return, and therefore was based upon blood being “expended” at its destination and not returning to the heart. Thus, the heart constantly produced blood. In the early 1600s, William Harvey wrote that the “energy” from the right and left ventricles (LVs) drove blood in a pulsatile manner to the lungs and the body, and that the lungs enabled inspired air to be drawn into the blood that returns to the left heart (1). While this was correct, Harvey’s theory of circulatory physiology did not connect the arteries to the veins. The “missing link” in the chain of the circulation was completed by Malpighi in the 1660s when he identified capillaries in the lungs and other organs.
With a better understanding of the cardiopulmonary circulation, scientists became interested in exploring the relationships between flow and pressure in blood vessels during respiration. In 1733, Reverend Stephen Hales, a veterinarian, observed the pulsatility of arterial blood through brass pipes that he inserted directly in the arteries of a live horse (2). Hales was the first to observe and measure blood pressure, and to show that this fell during inspiration and increased during expiration in healthy animals. In 1871, Kussmaul showed that the radial pulse was absent during inspiration but returned during expiration in patients with constrictive pericarditis due to tuberculosis (3). Pulsus paradoxus, which is in fact an exaggeration of normal physiology, was the first “pathologic” cardiopulmonary interaction to be described.
The Invasive Study of Cardiorespiratory Interactions: The Work of Cournand
A chapter on cardiopulmonary interactions, and our understanding of these, would be incomplete without discussion of the work of Cournand, Richards, and Forssmann who in the 1940s and 1950s conducted several hundred studies of cardiorespiratory physiology in healthy adults and in adults with cardiac failure, during spontaneous respiration and noninvasive ventilation. These studies were among the earliest to utilize cardiac catheterization and included measurements of intrathoracic (pleural) pressure, as well as intravascular pressures, stroke volume, and cardiac output. These comprehensive investigations provided important new insights into the
interrelationship between pleural pressure and cardiovascular performance, and provided explanations for many of the changes in hemodynamics that are observed during ventilation.
interrelationship between pleural pressure and cardiovascular performance, and provided explanations for many of the changes in hemodynamics that are observed during ventilation.
Pressure-Volume and Pressure-Flow Relationships
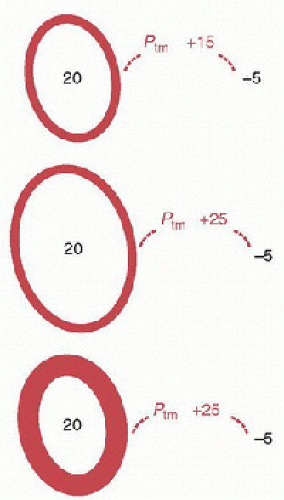
The study of the interactions between cardiovascular and pulmonary systems requires an understanding of pressure-volume and pressure-flow relationships applied to elastic structures, such as blood vessels, cardiac chambers, and alveoli. The fundamental property of an elastic structure is its ability to offer resistance to a distending or collapsing force and to return to its resting volume after the force has been removed. The extent to which a structure undergoes a change in volume depends on its compliance and the magnitude and direction of the pressure exerted across the wall—the transmural pressure (Fig. 70.1). The transmural pressure (Ptm) is equal to the difference between intra- and extracavitary pressures, where a positive transmural pressure distends the cavity and a negative transmural pressure causes the cavity to reduce in size.
Ptm = Pintracavitary – Pextracavitary
where Ptm is the transmural pressure, Pintracavitary the intracavitary pressure, and Pextracavitary the extracavitary pressure.
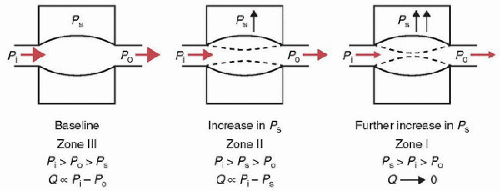
The physical principles that govern the flow of fluids (or air) through nonrigid conducting passages, such as vessels and airways, are derived from the general laws of hydrodynamics. Figure 70.2 shows that the flow (Q) through a collapsible tube depends on the following:
the “driving pressure” or “perfusion pressure,” which is the inflow pressure (Pi) minus the outflow pressure (Po);
the pressure surrounding the tube (Ps);
the transmural pressure (=Pi – Ps);
the compliance of the structure (which is the change in intravascular volume per unit change in pressure).
When the tube has a positive transmural pressure throughout, the tube is widely patent and Q is proportional to the pressure gradient Pi – Po. These conditions for the flow of fluid are also known as “zone III conditions.” With a constant Pi, Po, and compliance, as Ps increases, the transmural pressure decreases. As a result, the volume inside the tube decreases. Resistance to flow increases and flow is now proportional to the pressure gradient Pi – Ps. These conditions for the flow of fluid are also known as “zone II conditions.” As Ps increases further, the transmural pressure becomes negative, the tube collapses, and resistance to flow increases further, producing “zone I conditions.” The pressure at which Pi equals Ps is called the critical closing pressure. These different “zone” conditions may be created by changes not only in Ps but also as a result in changes in Pi, Po, and compliance. The physiologic significance of these concepts is that changes in Ps are analogous to changes in intrathoracic, intra-abdominal, and intravascular pressures with corresponding effects on systemic and pulmonary blood flow, resistance, and intravascular volume. While these concepts apply to both veins and arteries, there are special features of venous versus arterial flow, which are described below.
INTRATHORACIC PRESSURE AND RIGHT VENTRICULAR PRELOAD
Systemic venous return to the right heart is driven by a pressure gradient between the systemic venous circulation and the right heart and opposed by resistance to systemic venous return (RVR) or:
The mean systemic pressure (PMS) defined as the circulatory pressure if the heart were arrested (i.e., zero cardiac output) represents the inflow pressure (Pi) for venous return. PMS is a function of blood volume and capacitance of the systemic circulation (4). The systemic venous circulation is eighteen times more compliant than the systemic arterial circulation and therefore has much greater capacitance and holds the majority of intravascular volume (primarily within the splanchnic, splenic, and hepatic venous reservoirs) (5,6). The venous capacitance vessels, and not the arterial resistance vessels, are the principle determinant of PMS. The volume and pressure within the systemic arterial circulation has little impact on the mean systemic pressure (7).
Right atrial pressure (PRA) represents the outflow pressure (Po) for venous return. An increase in PRA causes systemic venous return and right ventricular output to decrease, unless there is a compensatory increase in PMS. Vasoconstriction of venous capacitance vessels, primarily mediated by adrenergic activation, angiotensin, and vasopressin, may increase PMS by reducing venous compliance and thereby mobilizing blood from the venous reservoirs to the central circulation (8,9,10,11,12).
Conversely, if venoconstriction increases the resistance to venous return without displacing venous blood volume from the periphery into the central circulation, the net effect will be to reduce venous return. Thus, the net effect of venoconstriction from sympatho-adrenergic discharge on venous return depends on whether it is accompanied by blood volume displacement into the central circulation (increasing PMS) or not (increasing RVR).
Over time an increase in PMS caused by increased central blood volume is complemented by the antidiuretic effects of vasopressin and by stimulation of the renin-angiotensina aldosterone system (13,14). As PMS decreases, venous return invariably decreases. Pharmacologic agents such as furosemide, nitric oxide donors, and angiotensin-converting enzyme inhibitors as well as pathologic conditions such as sepsis vasodilate venous capacitance vessels and cause a fall in venous return. (15,16,17,18). The fall in venous return leads to an equivalent fall in right ventricular output because the right heart can only eject the blood volume presented to it through systemic venous return.
Spontaneous Inspiration and Right Ventricular Preload
 In his landmark physiological investigations, Cournand demonstrated that the negative intrathoracic pressure (ITP) generated during spontaneous inspiration produced an increase in systemic venous return and hence right ventricular stroke volume and cardiac output (19). During spontaneous inspiration, the pleural pressure becomes negative and hence the transmural pressure of the right atrium increases.
In his landmark physiological investigations, Cournand demonstrated that the negative intrathoracic pressure (ITP) generated during spontaneous inspiration produced an increase in systemic venous return and hence right ventricular stroke volume and cardiac output (19). During spontaneous inspiration, the pleural pressure becomes negative and hence the transmural pressure of the right atrium increases.Right atrial transmural pressure = Right atrial intracavitary pressure – Pleural pressure
The reduction in pleural pressure increases right atrial compliance, causing right atrial pressure to fall and thereby increasing the pressure gradient for venous return. Also, during inspiration, the descent of the diaphragm increases intraabdominal pressure, decreasing the transmural pressure of the largest of venous reservoirs (20,21). Zone III conditions prevail in the abdomen if the abdominal transmural pressure remains positive (though reduced during inspiration). Hence, spontaneous inspiration in the euvolemic patient will displace abdominal blood volume into the central circulation and thereby contribute to venous return from the inferior vena cava. Alternatively, if diaphragmatic descent results in zero or negative abdominal transmural pressure (zone I and II conditions), the inferior vena cava becomes compressed and resistance to venous return is increased. Hence, a hypovolemic patient with decreased abdominal compliance will experience decreased venous return during inspiration. This is in contrast to venous return from the head and neck vessels, which are exposed to atmospheric pressure.
The increase in venous return as pleural pressure falls must not be limitless, otherwise during deep breathing the right heart would become overdistended. Guyton demonstrated that as right atrial pressure decreases venous return increases and then plateaus (Fig. 70.3). If the ITP becomes excessively negative during deep inspiration, the exaggerated negative ITP is transmitted to the caval veins as they enter the thoracic cavity. When the transmural pressure for the caval veins becomes negative at the thoracic inlets, the veins collapse limiting venous return (i.e., zone I and II conditions are created) (22). Further decreases in right atrial pressure have no effect on venous return because flow is now a function of the difference between mean systemic pressure and atmospheric pressure or abdominal pressure. When the outflow or downstream pressure is elevated, as in heart failure, the transmural pressure of the vena cava remains positive and venous return is limited by the outflow pressure (i.e., zone III conditions are created).
Positive Pressure Ventilation and Right Ventricular Preload
Cournand demonstrated that during PPV, there was a fall in systemic venous return and hence right ventricular stroke volume and cardiac output, which was proportionate to increases in the mean airway pressure.
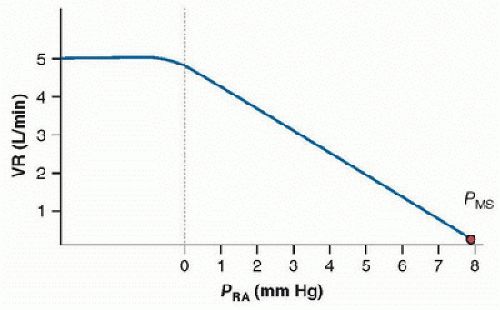 FIGURE 70.3. The relationship between right atrial pressure, mean systemic pressure, and venous return. Venous return (VR) increases as right atrial pressure (PRA) decreases and then plateaus as PRA falls below zero. The negative intrathoracic pressure is transmitted to the vena cava just outside the chest. As the transmural pressures for the vena cava become negative, they collapse at the thoracic inlet, creating zone I and II conditions, which limit or suspend venous return. Further decreases in PRA have no effect on venous return because flow is now a function of the difference between the mean systemic pressure (PMS) and atmospheric pressure or abdominal pressure. |
As ITP becomes increasingly positive, right atrial pressure increases and the pressure gradient for venous return decreases, therefore reducing systemic venous return. It may seem counterintuitive that an increase in right atrial pressure causes venous return to decrease because the right atrial pressure is considered a surrogate for right ventricular volume. However, it is changes in the right atrial transmural pressure and the determinants of the pressure gradient for venous return that determine venous return (23). Further, it is the right ventricular diastolic transmural pressure, not the right ventricular diastolic pressure, and ventricular compliance that determine right ventricular stroke volume (see Fig. 70.1). A noncompliant ventricle, or one surrounded by elevated ITP, requires a higher-than-normal intracavitary pressure to maintain an adequate end-diastolic volume, which in turn is needed to maintain adequate right ventricular stroke volume.
The extent to which an increase in ITP affects venous return depends in large part on the degree to which airway pressure is transmitted to the cardiac fossa, a function of respiratory mechanics, and on the adequacy of compensatory circulatory reflexes. Given the intricate relationship between right ventricular preload and cardiac output, it would follow that intravascular volume administration should restore cardiac output in the presence of high ITP when the intrinsic circulatory response does not suffice. This strategy, however, may not maintain cardiac output, due to changes in right ventricular afterload.
INTRATHORACIC PRESSURE AND RIGHT VENTRICULAR AFTERLOAD
If venous return were the only determinant of cardiac output during PPV, one would expect that, in the presence of normal myocardial function, volume expansion would preserve cardiac output under conditions of increasing ITP without limit. This is not necessarily the case. In a landmark study in healthy dogs that were ventilated with an end-expiratory pressure of 20 cm H2O, Henning observed that right ventricular stroke volume could not be normalized with volume administration despite complete restoration of right ventricular preload (24). The mechanism underlying this observation was that high ITP and lung volumes directly influenced pulmonary vascular resistance and right ventricular afterload and, hence, right ventricular output.
As lung volume is increased from residual volume to functional residual capacity, the radial traction provided by the pulmonary interstitium increases the diameter of the extraalveolar vessels. In addition, alveolar recruitment improves gas exchange and thus reverses hypoxic pulmonary vasoconstriction, further decreasing the resistance of extra-alveolar vessels. Despite a concomitant increase in the resistance of alveolar vessels, resulting from an increase in alveolar transmural pressure, the net effect is reduction of right ventricular afterload as lung volume approaches functional residual capacity. As lung volumes increase above functional residual capacity, the caliber of alveolar vessels decreases as alveolar transmural pressure continues to increase. Ultimately, alveolar pressure becomes greater than pulmonary venous pressure, creating zone II conditions and further increases in the resistance to blood flow (see Fig. 70.2). As lung volumes increase further, zone I conditions are created, as alveolar pressure rises above pulmonary arterial pressure, resulting in collapse of alveolar vessels and the cessation of blood flow to these areas. The net effect as lung volumes approach total lung capacity is an increase in pulmonary vascular resistance.
In the absence of cardiopulmonary disease, zone I conditions do not exist in the lung; however, they may be present in a variety of clinical scenarios. In addition to increases in alveolar pressure, zone I conditions may be created when pulmonary arterial pressures are low. In either case, zone I conditions are initially created in non-gravity-dependent regions of lung. Conversely, increases in alveolar pressure may not create zone I conditions if pulmonary venous pressures are elevated, as in left-sided heart failure. When pulmonary arterial and venous pressures exceed alveolar pressure, as in congestive heart failure or in the more gravity-dependent regions of the lung, zone III conditions predominate and flow is proportional to the pressure gradient between pulmonary arterial and pulmonary venous pressures. In zone III regions, alveolar distension leads to the propulsion of pulmonary blood flow and pulmonary venous return (25).
It is important to appreciate that it is not changes in alveolar pressure per se but rather changes in the alveolar distending pressure (i.e., alveolar transmural or transpulmonary pressure), lung compliance, and the resulting changes in lung volume during ventilation that affect pulmonary vascular resistance.
Alveolar (airway) Distending (transmural) Pressure = Alveolar (airway) Pressure – Pleural Pressure
Changes in the inflow and outflow pressures of the pulmonary circulation (i.e., pulmonary arterial and venous pressures, respectively) also determine the extent to which changes in lung volume alter pulmonary blood flow.
INTRATHORACIC PRESSURE AND LEFT VENTRICULAR PRELOAD
As discussed previously, spontaneous inspiration increases systemic venous return and right ventricular diastolic volume and pressure. As the right ventricle fills, the interventricular septum, which normally bows into the right ventricle because left ventricular pressures exceed those in the right ventricle, occupies a more neutral position between the two ventricles during diastole (Fig. 70.4). This effectively decreases left ventricular compliance and cavitary volume resulting in reduced stroke volume during inspiration (26,27). The mechanism by which the filling of one ventricle affects the filling of the other is diastolic ventricular interdependence, and contributes to pulsus paradoxus, the fall in systemic arterial pressure that occurs during spontaneous inspiration (28). Over the next few cardiac cycles, the increase in systemic venous return leads to an increase in left ventricular filling and output.
PPV may also limit left ventricular filling as a result of its effects on the right heart. Whether PPV predominantly affects
right ventricular preload or afterload depends on several factors including:
right ventricular preload or afterload depends on several factors including:
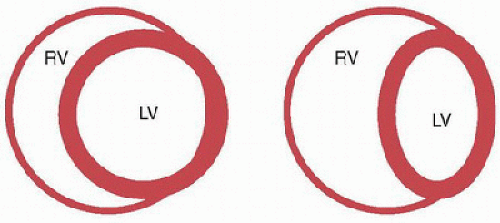 FIGURE 70.4. Ventricular interdependence. Cross-sectional view of ventricles from below. Under normal conditions (figure on left) the ventricular septum is oriented such that the left ventricle (LV) in its short axis is circular and the right ventricle (RV) is crescentic. Under conditions when the pressure in the RV is elevated (figure on right), the septum is displaced to the left, which decreases the effective compliance of the LV. |
the degree to which ITP is transmitted to the cardiac fossa (thereby reducing the pressure gradient for venous return);
the intravascular volume and the function of venous capacitance vessels;
the underlying right ventricular function;
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree