SETTING |
LIKELY ETIOLOGY |
APPROPRIATE INTERVENTION |
Early during mechanical ventilation |
Misplaced ET tube |
Confirm proper location by visualization and auscultation, CO2 detector |
|
Tension pneumothorax |
Physical examination, chest tube placement |
|
Hypovolemia |
Fluid bolus |
|
Auto-PEEP |
Reduce VE, increase expiratory time, bronchodilator, suction airway |
|
Hypoxemia |
Check ET placement, oximeter saturation, administer 100% O2 |
During chronic mechanical ventilation |
ET tube displacement |
Confirm proper ET placement by auscultation and chest radiograph |
|
Hypoxemia |
Confirm oxygenation with oximeter or ABG, increase FiO2 |
|
Tension pneumothorax |
Physical examination, chest tube placement |
|
Auto-PEEP |
Reduce VE, increase expiratory time, bronchodilator |
|
Mucus plugging |
Suction airway |
Post-central line placement/attempt |
Tension pneumothorax |
Physical examination, chest tube placement |
|
Tachyarrhythmia |
Withdraw intracardiac wires or catheters, try cardioversion/antiarrhythmic |
|
Bradycardia/heart block |
Withdraw intracardiac wires or catheters, try chronotropic drugs, temporary pacing |
During dialysis or plasmapheresis |
Hypovolemia Transfusion reaction
IgA deficiency: allergic reaction Hyperkalemia |
Fluid therapy Stop transfusion; treat anaphylaxis
Stop transfusion, treat anaphylaxis
Check K+, treat empirically if ECG suggests hyperkalemia |
During transport |
Displaced ET tube Interruption of vasoactive drugs |
Early identification using end-tidal CO2 Restart IV access |
Acute head injury |
Increased intracranial pressure (especially with bradycardia)
Diabetes insipidus: hypovolemia (especially with tachycardia) |
Lower intracranial pressure (ICP): hyperventilation, mannitol, 3% NaCl
Administer fluid |
Pancreatitis |
Hypovolemia |
Fluid administration |
|
Hypocalcemia |
Calcium supplementation |
After starting a new medicine |
Anaphylaxis (antibiotics) |
Stop drug, administer fluid, epinephrine, corticosteroids |
|
Angioedema (ACE inhibitors) |
|
|
Hypotension/volume depletion (ACE inhibitors) |
Volume expansion |
|
Methemoglobinemia |
Methylene blue |
Toxin/drug overdose Cyclic antidepressants |
Seizures/tachyarrhythmias |
Sodium bicarbonate |
β-Blocker/Ca2+ blocker |
Severe bradycardia |
Chronotropes, pacing, glucagon, insulin + glucose |
Organophosphates carbamates |
Severe bradycardia |
Decontamination, atropine, pralidoxime |
MAO inhibitor CO, cyanide |
Hypertension Hypoxia |
Drug removal Oxygen, sodium nitrite + sodium thiocyanate |
After myocardial infarction |
Tachyarrhythmia/VF Torsade de pointes |
DC countershock, lidocaine Cardioversion, Mg, pacing, isoproterenol, stop potential drug causes |
|
Tamponade, cardiac rupture |
Pericardiocentesis, fluid, surgical repair |
|
Bradycardia, AV block |
Chronotropic drugs, temporary pacing |
After trauma |
Exsanguination |
Fluid/blood administration, consider laparotomy-thoracotomy |
|
Tension pneumothorax |
Physical examination, chest tube placement |
|
Tamponade |
Pericardiocentesis/thoracotomy |
|
Abdominal compartment syndrome |
Measure bladder pressure, decompress abdomen |
Burns |
Airway obstruction |
Intubate, reintubate |
|
Hypovolemia |
Fluid administration |
|
Carbon monoxide |
100% O2 |
|
Cyanide |
Sodium nitrite-thiosulfate |
ABG, arterial blood gases; ACE, angiotensin-converting enzyme; AV, atrioventricular; DC, direct current; ECG, electrocardiogram; ET, endotracheal; PEEP, positive end-expiratory pressure; VF, ventricular fibrillation. |
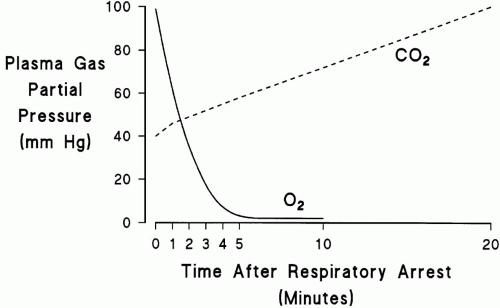
 to dramatically increase the rate of CO2 production. The net effect of these events is that life-threatening hypoxemia occurs long before significant respiratory acidosis.
to dramatically increase the rate of CO2 production. The net effect of these events is that life-threatening hypoxemia occurs long before significant respiratory acidosis.