INTRODUCTION AND EPIDEMIOLOGY
Detection of cardiac injuries is critical for patient survival. Penetrating cardiothoracic injury causes 25% of deaths immediately following trauma, and the majority of these fatalities involve either cardiac or great vessel injury.1 Cardiac injury may account for up to approximately 10% of deaths from gunshot wounds.2 The incidence of blunt cardiac injury has been reported to range anywhere from 8% to 71%.3 Suspect the diagnosis of cardiac and great vessel injury in a patient with chest, lower neck, epigastric, or precordial injury. Closely observe for evidence of hemodynamic instability, loss of circulating blood volume, electrocardiographic changes, cardiac tamponade, and hemothorax.
Penetrating cardiac injury results when a foreign object enters the body and pierces the pericardium or heart. Blunt cardiac injury results from physical forces acting externally on the body. Iatrogenic injuries to the heart are due to the invasive nature of procedures. All invasive cardiac procedures and therapies have the potential to cause trauma to both the pericardium and myocardium. Even noncardiac procedures like central lines placed into the internal jugular vein can lead to penetration of the pericardium, heart, and great vessels.4
PENETRATING CARDIAC TRAUMA
Most injuries occur from guns and knives.5 The injury usually involves only the free cardiac wall, but other structures can be injured, such as cardiac valves, chordae tendineae, papillary muscles, atrial or ventricular septum, coronary arteries, and conduction system (Table 262-1).6
Pericardial damage
Myocardial damage
Valvular injury
Coronary artery injury
Embolism
Infective endocarditis Rhythm or conduction disturbance |
Pericardial injury can result in acute tamponade. Rates of involvement of cardiac structures due to penetrating injuries to the right ventricle, left ventricle, right atrium, and left atrium are approximately 40%, 35%, 20%, and 5%, respectively.7,8 The right ventricle is at greatest risk due to its large anterior exposure on the chest wall. The right and left atria are less frequently involved due to their smaller surface area. Knives tend to involve a single chamber, producing a single slit-like defect that is often more amenable to medical and surgical therapy than gunshot wounds. Gunshot wounds can leave a spectrum of injury from multiple-chamber perforation to gaping defects depending on the caliber and velocity of the missile. Patients with stab wounds to the heart are 17 times more likely to survive than those with gunshot wounds.9 Atrial injuries are less common and generally less severe, whereas multichamber injuries are associated with higher mortality.
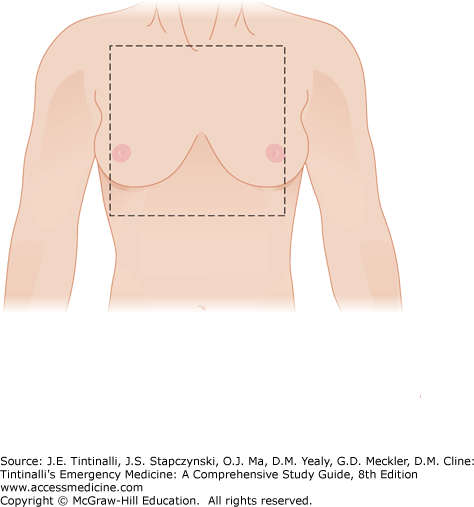
The anatomic “cardiac box” (Figure 262-1) is the area of the chest bounded by the sternal notch superiorly, the xiphoid process inferiorly, and the nipples laterally. Most stab wounds injuring the heart enter through this area. Gunshot wounds, however, may enter at regions well outside this area, so any penetrating injuries involving the thoracoabdominal region, back region, or any potential of transmediastinal trajectory place the heart at risk for injury.
The two conditions that can occur after penetrating cardiac injury are exsanguinating hemorrhage and cardiac tamponade. Pericardial defects that are large or remain open may present with hemothorax and clinical signs of blood loss that may progress to rapid exsanguination and death. If the pericardial wound seals itself, which is common with the linear defect in a stab wound, the result is intrapericardial hemorrhage that may progress to cardiac tamponade. The location of the wound will also determine the rate of accumulation. Right ventricular wounds tend to seal themselves more readily than right atrial wounds due to the thicker, more muscular walls of the ventricle. Atrial injuries may have subtle or no clinical findings initially; however, rapid clinical deterioration can occur. Injury to the coronary arteries is manifested by tamponade or myocardial ischemia.
Cardiac tamponade, characterized by the accumulation of pericardial fluid under pressure, can be found in up to 2% of penetrating trauma to the thoracoabdominal region and very rarely in blunt trauma. Up to 80% of myocardial stab wounds may develop cardiac tamponade. Gunshot wounds leave defects in the pericardium that are larger and more irregular than stab wounds. Gunshot wounds are therefore less likely to develop tamponade because it is more difficult for the pericardium to seal the defect.10
Blood in the pericardial cavity may be defibrinated by the lytic activity normally present in the pericardium, resulting in a hematoma that may inhibit normal myocardial function. Intrapericardial blood accumulation eventually causes elevated intrapericardial pressure, which leads to decreasing right and left ventricular filling. Catecholamines are released in this state as a compensatory mechanism that results in tachycardia and further elevated right-sided filling pressures. Once the distensibility limits from intrapericardial fluid are reached by the pericardium, the myocardial septum shifts toward the left side, which further reduces left ventricular filling and subsequent cardiac output. This downward spiral eventually produces irreversible shock and death. Even small amounts of blood (65 to 100 mL) can lead to an acute rise in intrapericardial pressure. Hypovolemic shock from other injuries may partially or completely compensate for the elevated right-sided pressures, resulting in normal or even low central venous pressures.
Cardiac tamponade is identified by the FAST exam. Without bedside US, cardiac tamponade can be deceptively difficult to diagnose as the body compensates for the hemodynamic effects by various mechanisms. The reduced cardiac output is offset by the increase in heart rate and increase in systemic vascular resistance. Often, the only clinical finding of pericardial tamponade is sinus tachycardia. Hypotension may be a sign of ominous decompensation and emergent need for surgical intervention if due to pericardial tamponade or systemic hypovolemia from other injuries. The classic finding of Beck’s triad of muffled heart sounds, hypotension, and distended neck veins is present in less than 10% of cases.2 Pulsus paradoxus, a substantial fall in systolic blood pressure during inspiration, and Kussmaul’s sign, an increase in jugular venous distention on inspiration, are not reliable signs and may only be found with moderate to severe tamponade.11 A more reproducible sign of cardiac tamponade is a narrowing of the pulse pressure, which along with elevation of the central venous pressure, is cardiac tamponade until proven otherwise. The narrowed pulse pressure is not sensitive, and its absence should never be used to exclude tamponade. Usually the jugular venous pressure is elevated and may be associated with venous distention of the neck veins, forehead, and even scalp.
The heart may have a retained missile from a direct injury or as a result of venous migration from another injury. These missiles, or the thrombus that may form as a result, may embolize into systemic or pulmonary arteries.12 Many patients with a retained cardiac missile are hemodynamically stable. The patients may do well with prolonged observation and no operative intervention.13 The treatment for missiles is highly variable, depending on missile size, shape, and location. Missiles that cause any hemodynamic instability or symptoms should be removed. A left ventricular missile that is free or partially exposed should be removed to prevent systemic embolization.13 Right-sided missiles may be removed or left alone because embolization to the pulmonary vascular bed usually has minimal consequences.14 Most intramyocardial and intrapericardial bullets and pellets are generally well tolerated and left in place. Embolized missiles need immediate removal because of the downstream ischemic effects of arterial occlusion. If a missile is embedded or adjacent to a coronary artery, it should be removed because of the potential for erosion and bleeding, which may lead to myocardial infarction and pericardial tamponade. Long-term effects of a retained missile may include bacterial endocarditis, thrombosis, embolization, and cardiac neurosis (many patients feel that they need any missile removed at all cost).13
Diagnostic and therapeutic percutaneous procedures may lead to many cardiovascular complications. Vascular (including extra- and intracardiac), valvular, and myocardial injuries are well-recognized complications of procedures such as central line placement, thoracentesis, chest tube placement, pericardiocentesis, percutaneous coronary catheterization, valvuloplasty, and electrophysiologic lab procedures.15
Perform pericardiocentesis if cardiac tamponade is identified on the FAST exam. This may be an effective temporizing measure. Perform ED thoracotomy in unstable patients with cardiac tamponade who may not survive transfer to an operating room.
Pericardiocentesis is a prelude to formal thoracotomy if there are inevitable delays to definitive surgery (i.e., transport to trauma center). Use of US guidance of the needle increases accuracy and is a class I American College of Cardiology/American Heart Association recommendation for critically injured patients. Detailed discussion is provided in chapter 34, “Pericardiocentesis.”
Patients in extremis but with electrical cardiac activity should be rapidly transported to the ED. Resuscitative ED thoracotomy can be lifesaving in carefully selected patients. The decision to perform an ED thoracotomy or alternately determine that resuscitation is futile must be made in short order under highly stressful circumstances.
Candidates for ED thoracotomy include penetrating chest trauma patients who are hemodynamically unstable and those who demonstrated signs of life (palpable pulse, a blood pressure, pupil reactivity, any purposeful movement, organized cardiac rhythm, or any respiratory effort) either in the field or ED but subsequently lost these signs of life. Resuscitative ED thoracotomy is likely to be futile in patients under the following clinical scenarios: (1) no pulse or blood pressure in the field; (2) asystole is the presenting rhythm and there is no pericardial tamponade; (3) prolonged pulselessness (>15 minutes) at any time; (4) other massive, nonsurvivable injuries; or (5) blunt traumatic cardiac arrest.
Exposure to the heart is accomplished by a left anterolateral thoracotomy. No matter where the penetrating injury occurs, use the left-sided approach initially. This approach allows for quick access to the pericardium and heart and exposure for aortic cross-clamping if necessary. Identify the left fourth or fifth intercostal space, which corresponds to an intercostal space below the male nipple or at the inframamillary fold in a woman. Make the incision in a single stroke through all layers down through the intercostal musculature from sternum to posterior axillary line. Use Mayo scissors to cut the remaining intercostal muscles. Insert a Finochietto retractor with the crank positioned near the bed. Open the retractor to allow exposure of the left thorax. Remove blood and inspect for brisk bleeding. Control intrathoracic hemorrhage with direct pressure, the application of appropriate pulmonary or vascular clamps, and direct suture ligation. Gently push the lung out of the field to expose the pericardium. To minimize the left lung obscuring the field, a right mainstem bronchus intubation may be performed.
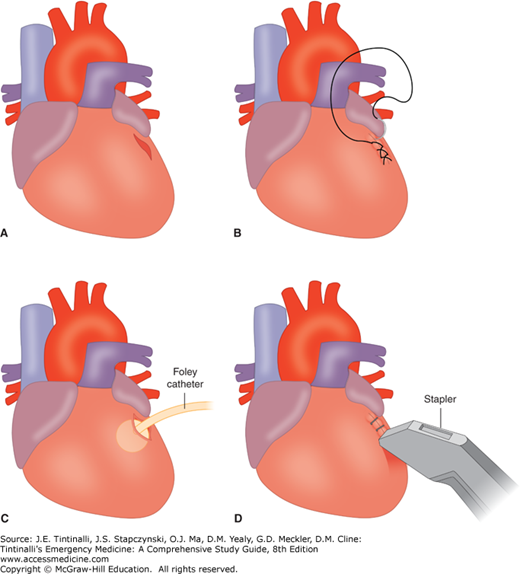
A distended, discolored, and often tense pericardial sac confirms cardiac injury. Perform a pericardiotomy by making a long incision from the apex of the heart to the root of the aorta just anterior to the phrenic nerve. Remove any blood and clot from the pericardial sac. Patients who do not have blood in the pericardium and do not exhibit cardiac activity may be declared dead at this point. If there is evidence of blood in the pericardium or cardiac activity, then expose the heart for inspection. After extruding the heart through the incision in the pericardium, identify the cardiac wound. One of several techniques may be used to control hemorrhage (Figure 262-2). Simple digital occlusion on top of the wound is the first maneuver to attempt initial hemostasis. Never insert a finger into the wound because this may extend the defect. The defect can then be stapled with regular skin staplers, which is a quick and easy method of temporary cardiac repair. Suturing of wounds is often technically difficult due to the dynamic nature of the myocardium, the slippery edges, the ease with which the sutures pull through the myocardium, inadequate lighting, and bloody field. Putting a Foley catheter into the defect and blowing up the balloon can fill a large circular defect. Gentle traction on the catheter will allow the balloon to seal the defect. A purse string suture can then be placed around the Foley catheter so that when the catheter is removed, tightening of the suture will seal the defect. Myocardial defects can be sutured with horizontal mattress sutures, but use pledgets to reinforce the repair and prevent cutting through the heart tissue.
FIGURE 262-2.
Temporary techniques to control bleeding of myocardial laceration. Emergency center management of injury to the epicardium. A. Stab wound to the left ventricle. B. Initially managed with either a continuous 4-0 polypropylene suture or interrupted sutures tied beneath the surgeon’s finger. C. Injuries that are more complex and cannot be managed in the ED can be temporized using a Foley catheter for ventricular injuries and either a Foley catheter and/or partial occluding clamp for atrial injuries. D. Because of concerns about surgeon needle stick during cardiorrhaphy on the beating heart, hemostasis can initially be achieved with the skin stapler. Care must be observed in avoiding ligation of coronary arteries.
Perform internal cardiac massage in the absence of coordinated cardiac function. Whatever type of repair is attempted, be careful and do not ligate major coronary vessels. If there is no evidence of injury to the left side or there is a high index of suspicion of right-sided injury, the ED thoracotomy incision can be extended to the right side. This is done by extending the incision across the sternum (“clam shelling”) in the same manner as the primary left-sided incision to gain access to the right side of the chest and allow for better exposure of the right ventricle and atrium. Patients with suspected intra-abdominal hemorrhage should have the descending aorta cross-clamped.
After resuscitative ED thoracotomy, if vital signs are regained, transfer the patient to the operating room for definitive repair. The survival rate for patients who make it to the operating room is 70% to 80% for stab wounds and 30% to 40% for gunshot wounds.
BLUNT CARDIAC TRAUMA
Up to 20% of all motor vehicle collision deaths are due to blunt cardiac injury, which can be sustained from any of the mechanisms listed in Table 262-2.16 Rapid deceleration is the most common mechanism responsible for most blunt cardiac injury followed by a direct blow to the precordium. Blunt cardiac injury results in a range of conditions from clinically silent transient dysrhythmias to cardiac wall rupture
Direct precordial impact Crush injury from compression between the sternum and spine Abrupt pressure fluctuations in the chest and abdomen Shearing from rapid deceleration or torsion causing a tear in the heart at a point of fixation (right atrium and vena cava) Injury from rib fracture fragments Hydraulic effect resulting in cardiac rupture Blast injury |
The most common reported injury is “myocardial or cardiac contusion.” These terms are nonspecific and have been used to report a wide range of injuries. Further, there is not a clear definition or a gold standard for testing, which makes the diagnosis and treatment difficult. The term blunt cardiac injury has replaced the terms cardiac contusion and myocardial contusion. Blunt cardiac injury can encompass cardiac dysfunction (diminished contractility in the absence of dysrhythmia or hemorrhage), dysrhythmias, specific injuries (septal rupture, valvular injuries, myocardial infarction), and cardiac rupture, the most devastating blunt cardiac injury.3,17,18
Blunt cardiac injury most often involves the right heart due to the anterior location of the right atrium and ventricle within the mediastinum. Injury often involves more than one chamber in over half of the reported cases.19
The pathologic changes seen in blunt cardiac injury typically include subendocardial hemorrhage and a much larger area of focal myocardial edema, interstitial hemorrhage, and myocytolysis with infiltrates of polymorphonuclear leukocytes. Additional myocardial injury may occur if there are concomitant intimal tears or compression from adjacent hemorrhage or edema. Myocardial injury may also be due to redistribution of coronary blood flow. Minor myocardial marker elevation or ECG abnormalities and dyskinesia or dysrhythmias resolve over time, usually within the first 24 hours.16 Blunt cardiac injury can lead to death from complex dysrhythmias, acute heart failure, cardiac-free wall rupture, or laceration of a coronary artery that causes extracardiac hemorrhage. Most of these lethal mechanisms result in death at the scene of the injury.20,21,22 Less severe injuries to the ventricular wall may lead to delayed necrosis and clinically manifest as delayed rupture days after admission. If a low-pressure chamber or coronary vein is injured, patients may survive until presentation to the hospital.23
Blunt cardiac injury can also result in rupture of an atrial or ventricular septum, resulting in shunting of blood and presentation similar to heart failure.24 Similarly, blunt cardiac injury can result in regurgitation of blood from a high-pressure chamber or artery into a lower-pressure chamber, such as with an acute valve dysfunction or papillary muscle injury.25,26 Valvular injury, as opposed to blunt myocardial injury, tends to worsen over time. The degeneration of the valve’s function appears to depend on its location. High-pressure valves like the aorta and mitral valves tend to manifest symptoms immediately or within the first few weeks, whereas lower-pressure valves like the pulmonic and tricuspid can be asymptomatic for years.16 Coronary arteries can have occlusion, dissection, or spasm that can manifest as immediate or delayed injury patterns. Coronary artery injury presents most often in a myocardial infarction pattern.27
Nonspecific signs and concomitant injuries will affect clinical presentation. These other findings in the trauma patient may make it difficult to determine whether symptoms stem from cardiac or other injuries. Specific signs of cardiac injury (e.g., distended neck veins or specific murmurs) may not be present if the patient is hypotensive from other injuries. Symptoms of cardiac injury may occur in a delayed fashion coincident with fluid resuscitation.
Commotio cordis, meaning “disturbance of the heart” in Latin, is sudden death as a result of blunt trauma to the chest wall. It often results from an innocent-appearing chest wall blow. It is the second most common cause of death in youth athletics following hypertrophic obstructive cardiomyopathy. Usually victims are young athletes who are struck in the chest by hard projectiles that are used in the particular sport. Sports using small dense projectiles like baseball, hockey, and lacrosse have the highest incidence of commotio cordis. The hardness of the impact object, location of impact, and velocity of the object impacts the risk of development of ventricular fibrillation. Commotio cordis blows are generally low impact, most of which are insufficient to cause any significant structural damage to the ribs, sternum, or heart. Commotio cordis is a primary electrical event resulting in the induction of ventricular fibrillation; it is a result of a blow that occurs 10 to 30 ms before the peak of the T wave, a time of vulnerability to ventricular fibrillation. Autopsy findings show normal cardiac anatomy with no evidence of injury. The overall survival rate is less than 15%, but due to increasing prevalence of automated external defibrillators being placed in sporting venues, survival rates may improve.28
The exact incidence of cardiac dysfunction (decreased contractility) in blunt cardiac injury is unknown. Further, the cause of dysfunction may be difficult to determine in the hypotensive, multiply injured trauma patient. Patients almost universally present with chest pain. The pain usually results from associated thoracic trauma (Table 262-3). Associated blunt injury to the lung can lead to a rise in pulmonary vascular resistance, which can result in a reduction in preload of the left ventricle. This, coupled with the reduced cardiac output of the involved right ventricle, can lead to hypotension.18,29 The damaged myocardial tissue may be a focus for both atrial and ventricular dysrhythmias, which may further produce decreased contractility and hemodynamic deterioration.
| Associated Injuries | Incidence of Finding in Patients with Blunt Cardiac Injury |
|---|---|
| Thoracic injury | |
| Chest pain | 18%–92% |
| Rib fracture | 18%–69% |
| Aortic or great vessel injury |