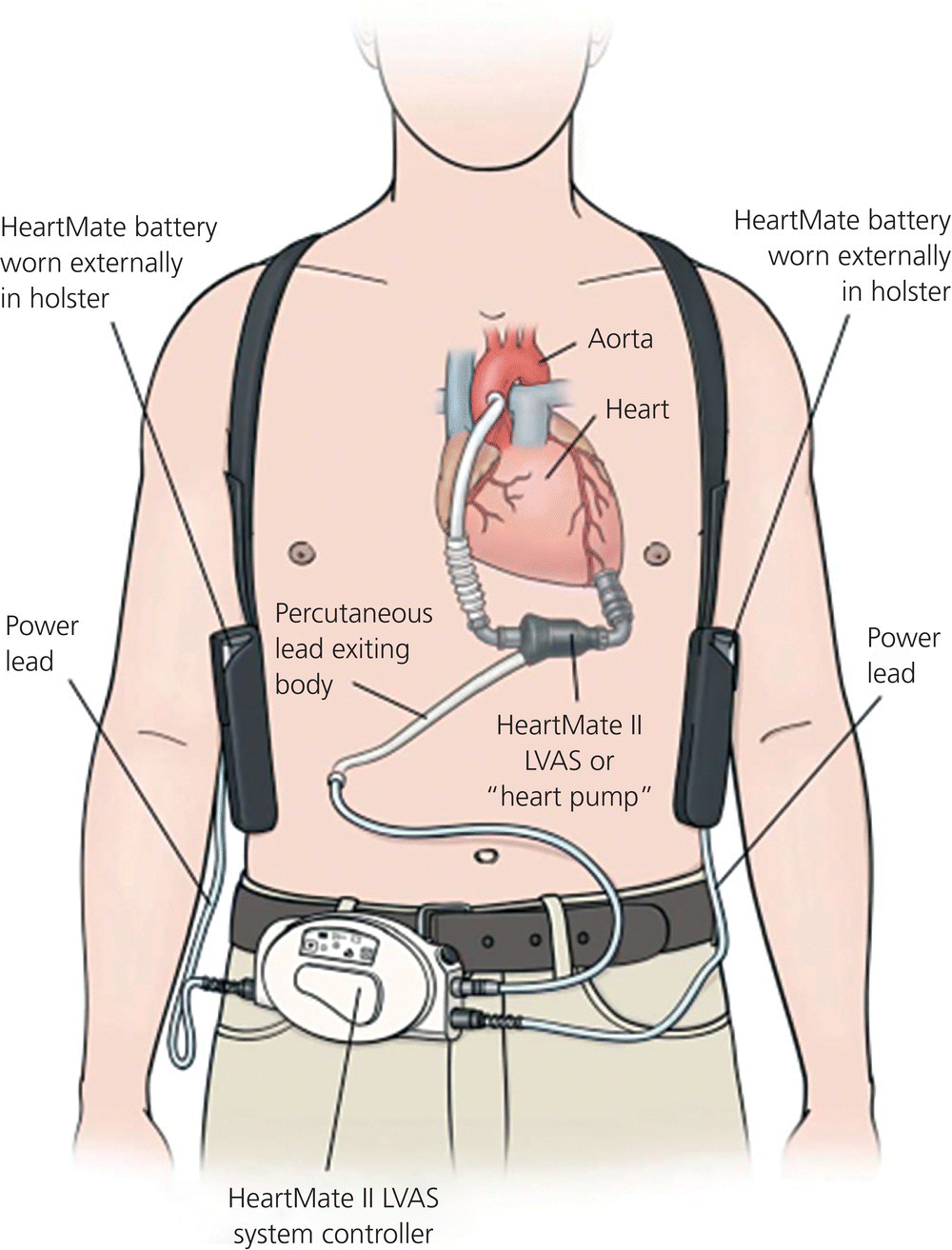
Chapter 15 T.J. Doyle The intraaortic balloon pump (IABP) is a mechanical device used in the stabilization of an acutely ill cardiac diseased patient. The EMS physician or critical care transport team will most commonly encounter the device during a patient transfer from a facility with limited or unavailable cardiac surgery capabilities to a tertiary care center. The role of the IABP is to provide cardiac stabilization until definitive care can be obtained. Goals of IABP therapy include decreasing cardiac afterload, augmenting diastolic perfusion pressure, and increasing coronary artery perfusion [1]. These efforts help to improve cardiac output that can in turn improve tissue perfusion. The decrease in afterload reduces the workload on the heart, and the improved coronary artery circulation can increase oxygen supply to the myocardium. Indications for IABPs most commonly encountered by EMS physicians are acute myocardial infarction, cardiogenic shock, ventricular aneurysm, left ventricular failure, valve or papillary muscle rupture, or a combination of these factors [2]. The patient is most commonly found in a catheterization lab, operating room, or coronary intensive care unit. The IABP catheter is placed via an incision in the lower extremity, inserted in the femoral artery, and then advanced into the thoracic aorta. The balloon should be placed 1–2 cm distal to the beginning of the subclavian artery, and must be above the branches of the renal arteries. If the balloon is not placed correctly, occlusion of coronary, subclavian, or renal arteries could occur [1]. On a chest x-ray, the tip of the catheter should be visible between the second and third intercostal spaces. When inflated, the balloon should not completely occlude the aortic lumen, as this can damage the aortic wall and blood components [1]. Most devices have different sized balloons for patients based on weight or height. It is important to ensure the appropriate balloon volume is being used. Absolute contraindications for an IABP include aortic dissection, abdominal aortic aneurysm, and aortic valve incompetence. Relative contraindications include bleeding disorders and atherosclerosis [1]. A patient with an IABP who requires interfacility transportation must be attended to by a specially trained team. In some cases, critical care paramedics, nurses, or physicians are trained to address IABP complications. Otherwise, it is vital that the transport team include a perfusionist or biomedical engineer. Intraaortic balloon pump function is critically dependent on timing. The balloon is cycled in conjunction with the cardiac cycle. It is important to remember that the balloon is inflated during diastole and deflated just prior to systole. While the balloon is inflated, blood is pushed both back toward the heart, as well as further down the aorta. The result is increased blood flow to coronary and carotid arteries, and increased systemic perfusion. The balloon is deflated very rapidly, and this rapid loss of volume reduces the pressure in the aorta. The result is that the left ventricle does not contract as hard as it would otherwise. Cardiac workload and myocardial oxygen demands are reduced. If the timing is not correct, these advantages are lost, and further harm to the patient may occur [1]. The IABP can use several different triggers for the inflation and deflation cycle. The most common modalities use the ECG or the arterial pressure waveform as a trigger. The IABP may also have an internal trigger in the event of cardiac arrest. Using arterial pressure as a trigger requires the patient to have an arterial catheter placed and connected to the balloon pump. Some IABP devices may have specialized fiberoptic connectors to measure arterial pressures. It is important to note that once a fiberoptic connector is placed and zeroed, it cannot be removed and connected to a different transport IABP. Another trigger modality needs to be used, such as ECG. Most devices have an “automatic” trigger mode, where the pump automatically switches between trigger modes if needed. An example would be a switch between ECG and arterial pressure modes if the ECG signal is lost. Most modern pumps can also compensate for arrhythmias such as atrial fibrillation and pacing modes [1]. With the trigger mode established, attention should focus on timing. Most patients are transported with a 1:1 frequency, where each cardiac cycle is assisted. In order to assess timing, it may be helpful to place the device in a 1:2 frequency to get a better picture of the arterial pressure waveform landmarks. For transport, the operator should ensure that the balloon is set to inflate at the dicrotic notch, and to deflate during the next isovolumetric contraction (IVC) phase. The dicrotic notch phase on the arterial pressure waveform represents aortic valve closure and diastole. Once the timing is correct, the device can be placed back into a 1:1 frequency and put in the “automatic” mode if available [1]. Potential complications include limb ischemia, compartment syndrome, aortic dissection, bleeding, thrombocytopenia and red blood cell destruction, gas embolus, infection, and cardiac decompensation from incorrect timing [1]. Special care must be taken when transferring a patient from one brand of IABP to another to enable transport. There may be a difference in the balloon size, and an adapter may be needed to connect a “Brand D” catheter to a “Brand A” IABP. The balloon size should be noted and adjusted on the pump if necessary. On arrival to a patient’s side, the transport team should examine the patient paying particular attention to the insertion site, as well as to the distal extremity. The insertion site should be examined for bleeding or protruding balloon. The catheter tubing should be examined for any blood or blood flecks. The distal extremity should be examined for ischemia. Catheter tubing should be examined for kinking. Any positive findings noted above should delay the transport until the situation can be corrected. Fresh ECG leads should be applied to the patient. The referring hospital balloon pump should not be disconnected or shut off until the transport pump is connected and tested. The transport balloon pump should be plugged into an outlet during this time and not run on battery power. The pump should also be plugged into an aircraft or ambulance power inverter during transport. Pure battery operation should be used only to transport the patient from the vehicle to his or her hospital destination. In the event of cardiac arrest, the IABP will lose all trigger modes, give a “trigger arrest” alarm, and then stop counterpulsation. If left unchanged, this could result in a thrombus formation. When cardiopulmonary resuscitation (CPR) is initiated, the IABP should be switched to “arterial trigger.” Effective CPR should allow for the IABP to function off the arterial pressures. In the event that arterial pressures are not sufficient, the IABP should be switched to an “internal trigger.” This last resort trigger provides asynchronous counterpulsation and will help prevent clot formation. “Internal trigger” mode should be stopped if there is a return of circulation and the ECG or arterial pressure mode is restarted [2]. In the event of IABP failure during transport, a large Luer-Lok syringe should be attached to the quick connector to aspirate the balloon for blood. If no blood is found, use air to inflate the balloon to the volume capacity of the balloon. Then quickly aspirate the air and deflate the balloon. Repeat 4–5 times every 5–10 minutes until the pump is repaired or replaced [1]. Ventricular assist devices (VAD) are surgically implanted pumps that are intended to assist one or both ventricles of the heart to pump when disease has diminished the heart’s native ability to do so. They are most often placed in patients with severe congestive heart failure. Devices include left ventricular assist devices (LVAD), right ventricular assist devices (RVAD), and biventricular assist devices (BIVAD). The most commonly placed device is the LVAD. The LVAD will have a cannula placed in the apex of the left ventricle with blood flow to the pump and a cannula placed into the ascending aorta with blood flow from the pump. Thus the device assists the ventricle in moving blood through the circulatory system [2]. Ventricular assist devices were first developed in the 1960s and the technology progressed during the 1970s and 1980s. Advances made them more portable, but the patient was still confined to the hospital. In the 1990s fully portable devices were developed that, for the first time, allowed VAD patients to be discharged from the hospital [3,4]. The devices are most commonly used as a bridge to cardiac transplantation, but they also may be used as a bridge to a reversible cardiac condition, or as a permanent therapy. There are two types of VAD patients: those with non-portable VADs, who would require critical care transport with a perfusionist, and those with portable VADs who may be living at home or in an assisted living facility. It is the second group of patients who are potentially encountered by EMS. Currently, there are four generations of VADs with features that can vary based on the generation and the particular device (Box 15.1). First-generation devices mimic the pumping action of the left ventricle via the use of diaphragms or pusher plates that cause blood to be sucked into the left ventricle and expelled into the aorta. This mechanism results in pulsatile blood flow. The patient will have a pulse and blood pressure that can be measured [4]. The pumps are powered by electricity and can be either electromechanical or pneumatic. Electromechanical pumps use an electromagnetic pusher plate to drive the blood, whereas pneumatic devices use air pressure to move the blood. Both devices require electrical power to function. Pneumatic devices may come with a hand pump in case of device failure [3]. Second-generation LVADs have continuous-flow rotary pumps. If the device only assists with the work of the left ventricle, the underlying function may result in a palpable pulse. If the LVAD is fully replacing the function of the ventricle, there may not be a palpable pulse. As with other technology advances, these devices offer advantages in size, ease of implantation, and durability. The number of moving parts has been reduced to one: the impeller. Second-generation LVADs are subdivided into devices with axial pumps and those with radial (centrifugal) pumps [3] (Figure 15.1). Figure 15.1 HeartMate II left ventricular assist device (LVAD). LVAS, left ventricular assist system. Source: Thoratec Corporation. Reproduced with permission of the Thoratec Corporation.
Cardiac procedures and managing technology
Intraaortic balloon pump
Special circumstances
Ventricular assist device
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





