Norwood with RV-PA conduit (Sano)
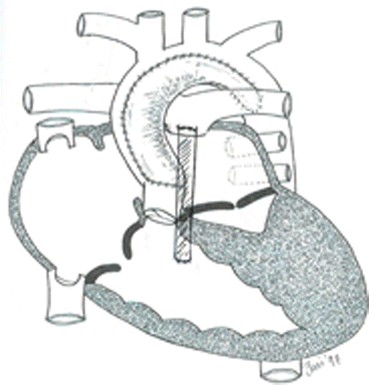
The Norwood procedure (Stage 1 palliation) as it applies to HLHS involves resection of the intra-atrial septum to allow unrestricted delivery of pulmonary venous blood to the systemic ventricle (RV) via the right atrium, a Damus-Kaye-Stansel procedure wherein the proximal main pulmonary artery and pulmonary valve are used to provide continuity between the RV and proximal aorta, an aortic arch reconstruction, and coarctectomy; autologous pericardium or graft material is used; either a modified Blalock-Taussig shunt (mBTS) or RV to main pulmonary conduit (Sano shunt) is constructed to provide pulmonary blood flow.
- 2.
Single ventricle physiology (SVP) is used to describe the situation wherein complete mixing of pulmonary venous and systemic venous blood occurs at the atrial or ventricular level, and one ventricle then distributes output to both the systemic and pulmonary beds (parallel circulation). As a result of this physiology, the ventricular output is the sum of pulmonary blood flow (Q p) and systemic blood flow (Q s); distribution of systemic and pulmonary blood flow is dependent on the relative resistances to flow (both intra- and extra-cardiac) into the two parallel circuits; oxygen saturations are the same in the aorta and the pulmonary artery.
This physiology can exist in patients with one well-developed ventricle and one hypoplastic ventricle as well as in patients with two well-formed ventricles such as truncus arteriosus and tetralogy of Fallot with pulmonary atresia.
- 3.
Common problems following the Norwood procedure include RV dysfunction with tricuspid regurgitation, residual aortic arch obstruction, and inadequate pulmonary blood flow secondary to shunt stenosis or patient growth. Differentiating poor systemic cardiac output and heart failure symptoms due to RV dysfunction, TR, and arch obstruction from low pulmonary blood flow symptoms due to shunt obstruction or inadequate shunt size can be difficult. Transthoracic echocardiography is the diagnostic procedure of choice.
Intraoperative Course
Questions
- 1.
How would you induce anesthesia for this patient? Maintenance technique?
- 2.
What monitoring would you use? Is an arterial catheter necessary?
- 3.
The surgeon would like to perform this procedure via a laparoscopic approach? Agree or disagree? Why?
- 4.
How would you treat hypotension (systolic BP <60 mmHg) and a reduction in SpO2 to 70 % occurring 30 min after insufflation of the abdomen with CO2?
Intraoperative Course
Answers
- 1.
Induction techniques that do not depress ventricular function and that prevent precipitous increases in SVR and PVR should be employed. Ketamine (1–2 mg/kg) in combination with a low dose of fentanyl (2–5 μg/kg) or remifentanil (0.5–1 μg/kg) and rocuronium are reasonable if an intravenous catheter is in place. In the absence of an intravenous catheter, oral or intramuscular premedication with ketamine and midazolam or inhalation of sevoflurane should not exceed 4 %, while maintaining spontaneous ventilation can be considered to facilitate placement. Anesthesia can be maintained with additional opioids delivered as a bolus or infusion in conjunction with isoflurane or sevoflurane.
Precipitous increases in SVR without an increase in single ventricle output result in diversion of output to the pulmonary circulation at the expense of the systemic circulation leading to reduced systemic oxygen delivery despite what initially appears to be “normal” BP and SpO2. Failure to recognize or prevent this scenario will ultimately result in cardiovascular collapse. In patients with depressed ventricular function and/or tricuspid regurgitation, judicious use of inotropic support (dopamine 3–5 μg/kg/min) may be necessary to maintain systemic oxygen delivery.
- 2.
There should be a low threshold to employ invasive blood pressure monitoring as meticulous attention to both systemic oxygen delivery (ABG analysis) and systemic perfusion pressure (beat to beat BP) is necessary.
- 3.
Laparoscopic procedures are increasingly being utilized, and it is important to recognize the effects of abdominal insufflation of CO2 on both hemodynamics and the reliability of end tidal CO2 as a surrogate measure of PaCO2. There is currently no strong evidence to support one approach over the other in terms of safety and efficacy.
- 4.
The first step is to rule out excessive abdominal insufflation pressures leading to compromise of venous return, elevation of systemic and pulmonary afterload, and compromise of alveolar ventilation. In the absence of hemodynamic or respiratory compromise from abdominal insufflation, a more careful analysis is necessary. In patients with SVP, hypotension associated with a fall in SpO2 should be assumed secondary to a fall in cardiac output (systemic oxygen delivery) due to the dependence of SaO2 on SvO2. A fall in SVR will cause a fall in BP, but a simultaneous fall in SaO2 is unlikely unless there is a significant pressure gradient across the shunt. There are two ways to increase cardiac output:
Increase HR.
Increase stroke volume:
Augment preload and increase end-diastolic volume.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





