Cardiac Anatomy
Steven M. Schwartz
Zdenek Slavik
Siew Yen Ho
KEY POINTS
 The use of the segmental approach to describe the complex congenital heart disease systems allows a clear form of communication of all essential elements of the cardiac anatomy.
The use of the segmental approach to describe the complex congenital heart disease systems allows a clear form of communication of all essential elements of the cardiac anatomy. There are two primary systems that are used to describe the anatomy of congenital heart disease, one of which relies primarily on cardiac segments and their attachments, and the other invokes presumed embryologic events. Many clinicians use a combination of the two systems as well as additional colloquialisms.
There are two primary systems that are used to describe the anatomy of congenital heart disease, one of which relies primarily on cardiac segments and their attachments, and the other invokes presumed embryologic events. Many clinicians use a combination of the two systems as well as additional colloquialisms. The cornerstones of segmental anatomy are identification of the morphologic atria and ventricles with description of their connections and alignments to each other and of the ventricles to the great arteries.
The cornerstones of segmental anatomy are identification of the morphologic atria and ventricles with description of their connections and alignments to each other and of the ventricles to the great arteries.Congenital heart disease is often simple to describe and understand, as in the case of an atrial or ventricular septal defect (VSD), but can also be quite complex and even forbidding to those not well versed in the subtleties of cardiac anatomy when there are more comprehensive defects. Significant controversies have persisted regarding the best overall approach to the nomenclature of complex congenital heart disease, and many cardiologists frequently use various shorthand designations or colloquialisms to describe these lesions, leading many noncardiologists to consider it an exercise in semantics or hairsplitting, best left to the pediatric cardiologist. In reality though, the intensive care physician must be familiar with the way in which such defects are described since anatomy often dictates physiology—physiology being the cornerstone of the clinical care of these patients. This chapter will review the common approaches to systematically and comprehensively categorizing the anatomy of congenital heart disease with the intended goal of familiarizing the pediatric critical care physician with the basic schemes used in assigning specific terms to specific defects. This chapter is not intended to resolve controversies where they exist, nor is it proffered as a comprehensive review of any one particular approach; rather, it will hopefully provide a practical framework for understanding how one goes about describing complex defects in an organized manner that helps the intensive care physician understand “how the blood goes ‘round’” and sets the basis for better communication with cardiology and cardiac surgery colleagues.
SEGMENTAL APPROACH TO CONGENITAL HEART DISEASE
Even though many common lesions can be described directly, the underlying basis for complete description of any congenital  heart lesion is a segmental approach. Segmental analysis of cardiac anatomy requires analysis of each cardiac segment, its morphology, and its relationship to adjoining cardiac segments. One can use this methodical approach to consider each part of the heart in turn: atria, ventricles, and great arteries. The connections between the segments (atrioventricular and ventriculo-arterial) are described, as are the atrial and ventricular septa, cardiac venous connections, and position of the heart within the thorax. Using a segmental approach, all congenital heart defects can be described in complete detail, and the physiology determined from the anatomy.
heart lesion is a segmental approach. Segmental analysis of cardiac anatomy requires analysis of each cardiac segment, its morphology, and its relationship to adjoining cardiac segments. One can use this methodical approach to consider each part of the heart in turn: atria, ventricles, and great arteries. The connections between the segments (atrioventricular and ventriculo-arterial) are described, as are the atrial and ventricular septa, cardiac venous connections, and position of the heart within the thorax. Using a segmental approach, all congenital heart defects can be described in complete detail, and the physiology determined from the anatomy.
 heart lesion is a segmental approach. Segmental analysis of cardiac anatomy requires analysis of each cardiac segment, its morphology, and its relationship to adjoining cardiac segments. One can use this methodical approach to consider each part of the heart in turn: atria, ventricles, and great arteries. The connections between the segments (atrioventricular and ventriculo-arterial) are described, as are the atrial and ventricular septa, cardiac venous connections, and position of the heart within the thorax. Using a segmental approach, all congenital heart defects can be described in complete detail, and the physiology determined from the anatomy.
heart lesion is a segmental approach. Segmental analysis of cardiac anatomy requires analysis of each cardiac segment, its morphology, and its relationship to adjoining cardiac segments. One can use this methodical approach to consider each part of the heart in turn: atria, ventricles, and great arteries. The connections between the segments (atrioventricular and ventriculo-arterial) are described, as are the atrial and ventricular septa, cardiac venous connections, and position of the heart within the thorax. Using a segmental approach, all congenital heart defects can be described in complete detail, and the physiology determined from the anatomy.There are two primary systems in use today for describing  complex cardiac defects. The merits of one system or the other have been the subject of numerous scholarly papers and debates (1,2), but in truth they have more similarities than differences, and many cardiologists and cardiac surgeons use terms derived from both systems at different times. Robert Anderson is the primary advocate of the concept of sequential segmental analysis emphasizing morphology, connections, and relations of segmental components as the basis for nomenclature. Richard and Stella Van Praagh, building substantially on work by Maurice Lev, have advocated a system also based on morphologic analysis of various segments, but with some significant differences in specific nomenclature, analysis, and definition of connections or junctions between segments. The use of this latter approach relies on cardiac embryology to understand the basic patterns of postnatal congenital heart disease and has inherent value, in that almost all lesions can be recognized to be the result of incomplete or inaccurate steps in the transition of the heart from the embryonic heart tube to the mature four-chamber heart. The value of the former system is that it allows description of any given situation, with no need to speculate about unobserved embryologic events.
complex cardiac defects. The merits of one system or the other have been the subject of numerous scholarly papers and debates (1,2), but in truth they have more similarities than differences, and many cardiologists and cardiac surgeons use terms derived from both systems at different times. Robert Anderson is the primary advocate of the concept of sequential segmental analysis emphasizing morphology, connections, and relations of segmental components as the basis for nomenclature. Richard and Stella Van Praagh, building substantially on work by Maurice Lev, have advocated a system also based on morphologic analysis of various segments, but with some significant differences in specific nomenclature, analysis, and definition of connections or junctions between segments. The use of this latter approach relies on cardiac embryology to understand the basic patterns of postnatal congenital heart disease and has inherent value, in that almost all lesions can be recognized to be the result of incomplete or inaccurate steps in the transition of the heart from the embryonic heart tube to the mature four-chamber heart. The value of the former system is that it allows description of any given situation, with no need to speculate about unobserved embryologic events.
 complex cardiac defects. The merits of one system or the other have been the subject of numerous scholarly papers and debates (1,2), but in truth they have more similarities than differences, and many cardiologists and cardiac surgeons use terms derived from both systems at different times. Robert Anderson is the primary advocate of the concept of sequential segmental analysis emphasizing morphology, connections, and relations of segmental components as the basis for nomenclature. Richard and Stella Van Praagh, building substantially on work by Maurice Lev, have advocated a system also based on morphologic analysis of various segments, but with some significant differences in specific nomenclature, analysis, and definition of connections or junctions between segments. The use of this latter approach relies on cardiac embryology to understand the basic patterns of postnatal congenital heart disease and has inherent value, in that almost all lesions can be recognized to be the result of incomplete or inaccurate steps in the transition of the heart from the embryonic heart tube to the mature four-chamber heart. The value of the former system is that it allows description of any given situation, with no need to speculate about unobserved embryologic events.
complex cardiac defects. The merits of one system or the other have been the subject of numerous scholarly papers and debates (1,2), but in truth they have more similarities than differences, and many cardiologists and cardiac surgeons use terms derived from both systems at different times. Robert Anderson is the primary advocate of the concept of sequential segmental analysis emphasizing morphology, connections, and relations of segmental components as the basis for nomenclature. Richard and Stella Van Praagh, building substantially on work by Maurice Lev, have advocated a system also based on morphologic analysis of various segments, but with some significant differences in specific nomenclature, analysis, and definition of connections or junctions between segments. The use of this latter approach relies on cardiac embryology to understand the basic patterns of postnatal congenital heart disease and has inherent value, in that almost all lesions can be recognized to be the result of incomplete or inaccurate steps in the transition of the heart from the embryonic heart tube to the mature four-chamber heart. The value of the former system is that it allows description of any given situation, with no need to speculate about unobserved embryologic events.Both of these systems are based on the principles (a) that specific cardiac chambers have unique, distinguishing characteristics that allow them to be clearly identified even when their left/right relationship to each other or connections to adjoining structures are altered or absent, and (b) that the precise nature of these relationships and connections should be carefully and fully described.
Atrial Situs
The terms “right” and “left” atria refer to specific, distinguishing morphologic characteristics of each atrium, not to the side  of the heart on which they are located or their accompanying venous or ventricular connections. Features that identify the right atrium include (a) a blunt atrial appendage with a wide connection to the smooth-walled part of the atrium, (b) the limbus of the fossa ovalis, and (c) remnants of the valves of the sinus venosus such as the Eustachian valve of the coronary sinus. The left atrium is characterized by (a) a hooked appendage with a more narrow connection to the smooth-walled portion of the chamber, (b) the flap valve of the fossa ovalis, and (c) no remnants of venous valves. Note that venous connections are not used to designate the morphologic right and left atria since these connections can be variable in congenital heart disease (i.e., total anomalous pulmonary venous return, unroofed coronary sinus, bilateral superior vena cava). Additionally, the atrial appendages may be juxtaposed, wherein the morphologic left atrium has an appendage with the characteristics of a right atrial appendage or vice versa, so that use of this feature to identify which atrium is which is of limited value in certain cases.
of the heart on which they are located or their accompanying venous or ventricular connections. Features that identify the right atrium include (a) a blunt atrial appendage with a wide connection to the smooth-walled part of the atrium, (b) the limbus of the fossa ovalis, and (c) remnants of the valves of the sinus venosus such as the Eustachian valve of the coronary sinus. The left atrium is characterized by (a) a hooked appendage with a more narrow connection to the smooth-walled portion of the chamber, (b) the flap valve of the fossa ovalis, and (c) no remnants of venous valves. Note that venous connections are not used to designate the morphologic right and left atria since these connections can be variable in congenital heart disease (i.e., total anomalous pulmonary venous return, unroofed coronary sinus, bilateral superior vena cava). Additionally, the atrial appendages may be juxtaposed, wherein the morphologic left atrium has an appendage with the characteristics of a right atrial appendage or vice versa, so that use of this feature to identify which atrium is which is of limited value in certain cases.
 of the heart on which they are located or their accompanying venous or ventricular connections. Features that identify the right atrium include (a) a blunt atrial appendage with a wide connection to the smooth-walled part of the atrium, (b) the limbus of the fossa ovalis, and (c) remnants of the valves of the sinus venosus such as the Eustachian valve of the coronary sinus. The left atrium is characterized by (a) a hooked appendage with a more narrow connection to the smooth-walled portion of the chamber, (b) the flap valve of the fossa ovalis, and (c) no remnants of venous valves. Note that venous connections are not used to designate the morphologic right and left atria since these connections can be variable in congenital heart disease (i.e., total anomalous pulmonary venous return, unroofed coronary sinus, bilateral superior vena cava). Additionally, the atrial appendages may be juxtaposed, wherein the morphologic left atrium has an appendage with the characteristics of a right atrial appendage or vice versa, so that use of this feature to identify which atrium is which is of limited value in certain cases.
of the heart on which they are located or their accompanying venous or ventricular connections. Features that identify the right atrium include (a) a blunt atrial appendage with a wide connection to the smooth-walled part of the atrium, (b) the limbus of the fossa ovalis, and (c) remnants of the valves of the sinus venosus such as the Eustachian valve of the coronary sinus. The left atrium is characterized by (a) a hooked appendage with a more narrow connection to the smooth-walled portion of the chamber, (b) the flap valve of the fossa ovalis, and (c) no remnants of venous valves. Note that venous connections are not used to designate the morphologic right and left atria since these connections can be variable in congenital heart disease (i.e., total anomalous pulmonary venous return, unroofed coronary sinus, bilateral superior vena cava). Additionally, the atrial appendages may be juxtaposed, wherein the morphologic left atrium has an appendage with the characteristics of a right atrial appendage or vice versa, so that use of this feature to identify which atrium is which is of limited value in certain cases.Once the morphology of the atria has been identified, the atrial arrangement or situs can be defined. Atrial situs refers to the arrangement of the morphologic right and left atria within the heart. Situs solitus is the normal arrangement, wherein the right atrium is to the right and the left atrium is to the left. Situs inversus is the opposite arrangement; the right atrium is to the left and the left atrium is to the right. A major difference between the Anderson and Van Praagh approaches to nomenclature concerns the recognition of the possibility of atrial isomerism. Atrial isomerism is defined as having two morphologically left or right atria. Van Praagh contends that having two right or left atria is an impossibility and that atrial situs in all hearts can be described as solitus, inversus, or ambiguus, in which ambiguus refers to the situation where separate right and left atria cannot be differentiated (3). Using this approach, anatomic variants that would be considered cases of atrial isomerism are more generally defined in terms of heterotaxy syndromes since there are often extracardiac abnormalities of laterality that coexist with the congenital heart disease. Polysplenia syndrome is thus often used to mean the same thing as left atrial isomerism, and asplenia syndrome is often used to mean the same atrial arrangement as right atrial isomerism. Anderson argues that this approach is at times inaccurate, since not all patients with congenital heart disease congruent with polysplenia are actually polysplenic, and not all with cardiac anatomy consistent with asplenia are asplenic. Rather, the Andersonian system suggests that atrial situs can be identified in all cases as usual or solitus, mirror image or inversus, and right isomerism or left isomerism (4).
As suggested earlier, the assignment of atrial situs can be assisted by observation of other asymmetrically distributed structures, and the identification of atrial isomerism can lead to investigations for malposition of noncardiac structures. The bronchi can be particularly helpful in identifying atrial situs since the right and left bronchi have characteristic features readily seen on chest x-ray and since bronchial situs almost always reflects atrial situs (5). Specifically, the right mainstem bronchus is shorter, more vertically oriented and is located directly behind the mediastinal segment of the right pulmonary artery. The left mainstem bronchus is longer, more horizontally oriented and below the left pulmonary artery. When the atria are inverted, the left-sided bronchus will usually have the orientation of a typical right bronchus and the left lung may contain three lobes. The right-sided bronchus will then have a typical left mainstem bronchus anatomy. Isomeric arrangement of the atria usually is reflected by a bilaterally symmetrical bronchial arrangement of either a right or a left type. In heterotaxy syndromes (atrial isomerism), abdominal structures in addition to the spleen can be abnormally distributed. Right atrial isomerism or asplenia can be thought of as bilateral right-sidedness and can be associated with a midline liver and intestinal malrotation. Left atrial isomerism or polysplenia syndrome can be thought of as bilateral left-sidedness and is also associated with an intestinal malrotation.
The finding of atrial isomerism is also typically accompanied by certain predictable, associated intracardiac defects. Right atrial isomerism or asplenia syndrome usually includes anomalies of pulmonary venous return, atrioventricular canal-type defects, double outlet right ventricle (DORV), and transposition (6,7,8). Left atrial isomerism tends to be more commonly associated with abnormalities of systemic venous connections (interrupted inferior vena cava) and DORV or normally related great arteries. Polysplenia can also be associated with atrioventricular canal defects and some abnormalities of pulmonary venous drainage, but usually not to the degree these are associated with asplenia. These associations are important for the cardiologist in that they prompt complete assessment for these types of lesions, but also for the intensivist in terms of providing a mental framework for understanding and description of these complex anatomic variants.
The Atrial Septum
The atrial septum, in its usual state, contains a foramen ovale on the right side and the flap valve of the foramen on the left. The foramen, when patent, allows right-to-left shunting of blood when the right atrial pressure exceeds the left, but is closed by the flap valve when left atrial pressure is higher. The foramen ovale is the remnant of the ostium secundum, and therefore, atrial septal defects (ASDs) in this area are referred to as secundum ASDs. The foramen primum is the embryologic opening between the early atrial septum (septum primum) and the area where the endocardial cushions ultimately form. The foramen primum is closed by endocardial cushion tissue, thus making primum ASDs a common component of atrioventricular canal (endocardial cushion) defects. The least common type of ASD is the sinus venosus ASD, which is found in the superior part of the atrial septum where the sinus venosus is incorporated into the embryonic atrium. This type of defect is often associated with partial anomalous venous connection of the right upper pulmonary vein.
Ventricular Morphology and Topology
As with the atria, the right and left ventricles are identified by certain constant morphologic patterns, not by their location within the heart or the body. The morphologic right ventricle (a) is heavily trabeculated, (b) contains the moderator band of the septum, and (c) has septal attachments of the associated atrioventricular valve. The left ventricle (a) is smooth walled, (b) is more bullet shaped, and (c) has no septal attachments of the atrioventricular valve. When there are two distinct atrioventricular valves, the tricuspid valve (TV) is always associated with the morphologic right ventricle, and the mitral valve is always associated with the left ventricle.
The topology of the ventricles refers to the spatial anatomy of the ventricle with regard to the inflow and outflow. Determination of the “handedness” of a ventricle is made by considering the relationship of the inflow and outflow tracts of the ventricle when looking at the septal surface (Fig. 68.1). One can imagine placing the palm of the hand on the septal
surface of the morphologically right ventricle with the thumb extended toward the inflow and the fingers pointed toward the outflow. If this can be accomplished with the right hand, the ventricle is said to be “right handed”; if it can be accomplished with the left hand, the ventricle is “left handed.” In general, a normal heart is said to have right-handed topology, but this terminology does not describe or identify the morphology of the ventricle, nor does it describe the location within the heart, although certain anatomic and morphologic arrangements are generally associated with specific handedness for the morphologic right and left ventricles.
surface of the morphologically right ventricle with the thumb extended toward the inflow and the fingers pointed toward the outflow. If this can be accomplished with the right hand, the ventricle is said to be “right handed”; if it can be accomplished with the left hand, the ventricle is “left handed.” In general, a normal heart is said to have right-handed topology, but this terminology does not describe or identify the morphology of the ventricle, nor does it describe the location within the heart, although certain anatomic and morphologic arrangements are generally associated with specific handedness for the morphologic right and left ventricles.
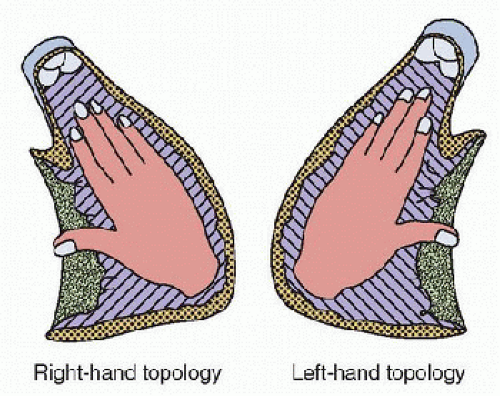 FIGURE 68.1. Ventricular topology is determined by imagining the palmer surface of the hand on the septal surface of the morphologically right ventricle with the thumb pointing toward the inlet and the fingers pointing toward the outlet. Right-hand topology is present if this can be accomplished with the right hand. Left-hand topology is present if this alignment requires use of the left hand. (From Anderson RH. Nomenclature and classification: Sequential segmental analysis. In: Moller JH, Hoffman JIE, eds. Pediatric Cardiovascular Medicine. Philadelphia, PA: Churchill Livingstone, 2000:263-74, with permission.) |
Atrioventricular Connections, Junctions, and Alignments
The exact nature of the atrioventricular relationship can take many forms. Usually, there are two separate atrioventricular valves, one leading into each ventricle. Furthermore, the right atrium usually opens into the morphologic right ventricle, and the left atrium into the left ventricle. Of course, in complex congenital heart disease, this is not always the case and as such, it is important to precisely describe the way in which this part of the heart is arranged. For the purpose of clarity, it is helpful to consider both the type of atrioventricular connection and the mode of connection. The type of atrioventricular connection or alignment refers to the anatomic concordance or discordance of the atrium and ventricle. A normal heart has concordant atrial and ventricular connections or alignments, in that the morphologic right atrium opens to the morphologic right ventricle, and the left atrium does likewise with the left ventricle. A discordant connection or alignment is one in which the morphologic right atrium opens into the morphologic left ventricle and vice versa. When there is atrial isomerism or when both atria open into one ventricle, one can describe the relationship as ambiguous and refer to the topology of the ventricles (Fig. 68.2) or to the looping pattern. Also, a single common atrioventricular valve that guards the opening between right and left atria above and right and left ventricles below can still be referred to in terms of concordant and discordant connections or alignments.
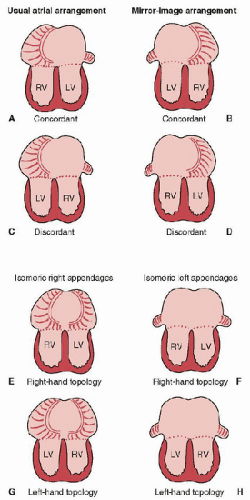 FIGURE 68.2. The atrioventricular connection can be described as concordant or discordant when there are two morphologically different atria (A-D). When there is atrial isomerism, the connection can be referred to by identifying the atrial morphology followed by the ventricular topology (E-H). RV, Right ventricle; LV, Left ventricle. (Modified from Anderson RH. Nomenclature and classification: Sequential segmental analysis. In: Moller JH, Hoffman JIE, eds. Pediatric Cardiovascular Medicine. Philadelphia, PA: Churchill Livingstone, 2000:263-74.) |
The mode by which the atria open into the ventricles must also be described accurately. This refers to the precise morphology of the atrioventricular valve or valves. When there are two atria, two atrioventricular valves, and two ventricles, the TV is always associated with the morphologic right ventricle, and the mitral valve is always associated with the morphologic left ventricle. When one of the valves is atretic and/or the ventricle is hypoplastic, there are still considered to be two atrioventricular valves in terms of assignment of anatomic diagnosis (e.g., tricuspid atresia or hypoplastic left heart). Nomenclature
is more complex when there is a single, common atrioventricular valve as in an atrioventricular canal type defect where the two valves are not fully formed and distinct, or when there is a double inlet connection (both atrioventricular valves open into the same ventricle). In these types of anatomic situations, the atrioventricular valves are often referred to as left and right. The basis of this argument is that since neither is a properly formed valve, use of the names “tricuspid” and/or “mitral” is technically inaccurate, and thus, referring to the valves on the basis of their respective position within the heart is most correct (1). Others have suggested that even under these circumstances one can identify the valves as mitral and/or tricuspid and should thus do so (2).
is more complex when there is a single, common atrioventricular valve as in an atrioventricular canal type defect where the two valves are not fully formed and distinct, or when there is a double inlet connection (both atrioventricular valves open into the same ventricle). In these types of anatomic situations, the atrioventricular valves are often referred to as left and right. The basis of this argument is that since neither is a properly formed valve, use of the names “tricuspid” and/or “mitral” is technically inaccurate, and thus, referring to the valves on the basis of their respective position within the heart is most correct (1). Others have suggested that even under these circumstances one can identify the valves as mitral and/or tricuspid and should thus do so (2).
The nature of the atrioventricular connection can be further complicated when an inlet VSD is present. These types of VSDs often involve override or straddle the atrioventricular valve. Override occurs when the annulus of the atrioventricular valve overrides the ventricular septum and thus allows the atrium associated with the overriding valve to empty into both ventricles. A straddling atrioventricular valve is one in which the chordal attachments of the valve cross the plane of the septum to attach to the contralateral ventricle. The left atrioventricular valve, for example, may have chordal attachments to the right side of the ventricular septum. Override and straddle are not mutually exclusive, and straddle is the far more complex problem to repair, leading to consideration of single ventricle-type palliation when significant straddle is present.
Ventricular Arrangements
Using the sequential segmental approach of Anderson, once the morphologic right and left atria and ventricles have been identified, and once the nature of the atrioventricular connection has been ascertained, the relationships between the atria and ventricles can be described by referring to concordance, discordance, and topology as needed (9). Single ventricles are described with reference to morphology of the ventricle. The nomenclature system proposed by Van Praagh is based on an embryologic approach to segmental anatomy (10). Understanding this system requires one to have basic familiarity with the transition of the heart from the straight heart tube of the early embryo to the mature, four-chambered heart of the fetus and newborn (Fig. 68.3). The heart tube contains the progenitor areas for all four cardiac chambers. The most caudal areas of the heart tube, the sinus venosus and atrium, are destined to become the right and left atria. The embryologic atrium leads directly into the embryonic ventricle, which is the future left ventricle. Moving more rostrally, the ventricle opens into the bulbus cordis, destined to become right ventricle, which then gives rise to the outlet of the heart, the conus arteriosus and arterial trunk. Transition from the straight heart tube requires the tube to loop, usually with the apex of the loop being rightward, referred to as a dextro or d-loop. The apex occurs at the junction of the ventricle and bulbus cordis. When d-looping occurs, the embryologic ventricle ends up leftward of the bulbus cordis, thus placing the left ventricle to the left of the right ventricle. When looping occurs abnormally, with the apex of the loop to the left, one is faced with a situation in which the morphologic left ventricle ends up rightward of the morphologic right ventricle. This arrangement is referred to as a levo or l-loop.
Obviously, it is impossible to replay the exact sequence of embryologic development when it is necessary to make a precise anatomic diagnosis in a fetus or newborn. The power of this particular nomenclature system, however, lies in its ability to predict comprehensive patterns of abnormalities since much congenital heart disease represents incomplete or improperly finished incidents in embryologic heart development. From a practical standpoint then, a heart in which the morphologic right ventricle is on the right and the morphologic left ventricle is on the left side of the heart is d-looped, and a heart in which the morphologic right ventricle is on the left and the morphologic left ventricle is on the right is l-looped. This nomenclature system further requires that the lower cardiac chamber have an inlet from an atrium to be called a ventricle. This inlet can be atretic, but must be present. Lower chambers with an outlet but not an inlet are referred to as outlet chambers; lack of both an inlet and an outlet is the hallmark of a trabecular pouch. A lower chamber with an inlet but no outlet is still considered a ventricle. Position of outlet chambers and/or trabecular pouches combined with the morphology of the ventricle can be used to determine the looping pattern in single ventricle anatomy. Recognize, however, that hypoplastic left heart syndrome, for example, has two anatomic ventricles so that it can be named using a system for biventricular hearts, even though the physiology is that of a single ventricle.
The Ventricular Septum
The ventricular septum is normally composed of an inlet portion formed by endocardial cushion tissue, a trabecular or muscular portion that represents the muscular area between the ventricle and bulbus cordis after looping is completed (sometimes described as having sinus and trabecular portions), and the outflow or conal portion of the septum. Malalignment defects such as those that occur in tetralogy of Fallot (TOF) or interrupted aortic arch are generally located in this area because they represent failure of proper alignment of the portions of the ventricular septum. The junction between all of these parts is the membranous septum, the most common site of VSDs. Inlet defects occur posteriorly in the plane of the atrioventricular valves, muscular VSDs occur anywhere in the trabecular septum, and outlet VSDs are found in the cono-truncal region. It is important to understand that the description of VSD location in many types of complex defects is more related to the position of the great arteries than to the VSD itself. In other words, two hearts with VSDs in the outlet septum may be called by different terms (e.g., subaortic vs. subpulmonary) based on the arrangement of the great arteries.
The Great Arteries
The great arteries have relationships with the ventricles and with each other. The ventricular relationships are defined and determined by outflow tract anatomy and alignments. The relationship of one great artery to the other is considered in terms of anterior-posterior and left-right positioning. Normally related great arteries occur when there are concordant ventriculo-arterial connections with the aorta posterior and slightly rightward of the pulmonary artery (Fig. 68.4A). In ventriculo-arterial concordance, the aorta arises from the morphologic left ventricle and the pulmonary artery arises from the morphologic right ventricle. Ventriculo-arterial discordance occurs when the aorta arises from the morphologic right ventricle and the pulmonary artery arises from the morphologic left ventricle. Concordance and discordance can thus be considered to be synonymous with normally related and transposed great arteries, respectively (11,12), although some have suggested the anterior-posterior relationship of the great arteries is the most definitive feature of transposition, at least from an historical perspective (1,13). Given the possibilities for confusion therefore, some have argued that the terms “concordant” and “discordant” should be used to
describe all situations in which two ventricles connect individually to two great arteries (1). When there is a double outlet ventriculo-arterial connection, one of the ventricles gives rise to both great arteries. In this case, or in the case of only a single great artery arising from a discordant ventricle, the artery with improper orientation can be said to be malposed (11,12). The term malposition can also be used to describe anterior-posterior malposition in the presence of ventriculoarterial concordance. The anatomic arrangement of the great arteries should be distinguished from their physiologic orientation, in that deoxygenated blood can course from right atrium to morphologic left ventricle to transposed pulmonary artery, resulting in a physiologically normal situation.
describe all situations in which two ventricles connect individually to two great arteries (1). When there is a double outlet ventriculo-arterial connection, one of the ventricles gives rise to both great arteries. In this case, or in the case of only a single great artery arising from a discordant ventricle, the artery with improper orientation can be said to be malposed (11,12). The term malposition can also be used to describe anterior-posterior malposition in the presence of ventriculoarterial concordance. The anatomic arrangement of the great arteries should be distinguished from their physiologic orientation, in that deoxygenated blood can course from right atrium to morphologic left ventricle to transposed pulmonary artery, resulting in a physiologically normal situation.
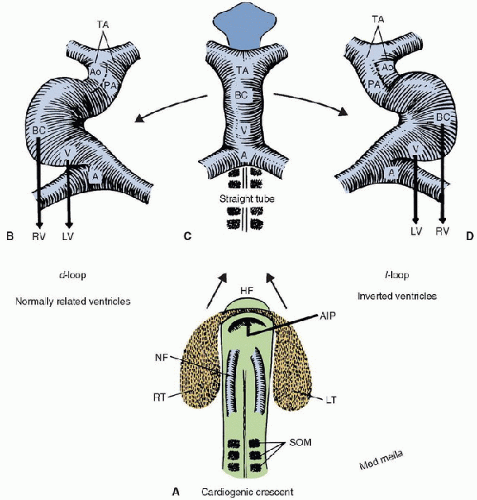 FIGURE 68.3. The embryologic cardiac crescent (A) contains the early precursors of the straight heart tube (B). The tube usually loops with the apex to the right forming a d-loop (C) and bringing the future right ventricle to the right of the future left ventricle. When the apex of the loop is to the left, or l-looped (D), the morphologic right ventricle ends up leftward of the morphologic left ventricle, so-called ventricular inversion. HF, Head fold; AIP, Anterior intestinal portal; NF, Neural fold; RT, Right side of cardiac crescent; LT, left side of cardiac crescent; som, somites; A, atrium; V, ventricle (future left ventricle); BC, Bulbus cordis (future right ventricle); TA, Truncus arteriosus; LV, Left ventricle; RV, Right ventricle; Ao, Aorta; PA, Pulmonary artery. (From Van Praagh R, Weinberg PM, Matsuoka R, et al. Malpositions of the heart. In: Adams FH, Emmanouilides GC, eds. Moss’ Heart Disease in Infants, Children and Adolescents. 3rd ed. Baltimore, MD: Williams & Wilkins, 1983:422-58, with permission.) |
Using the nomenclature system proposed by Anderson, each great artery is assigned to the ventricle to which it is more than 50% committed (1). This approach offers the benefit of simplicity that comes with the intuitiveness of the nomenclature. DORV, for example, occurs when both the great arteries are more than 50% committed to the right ventricle. One must also then describe the relationship of the great arteries to each other, such as DORV with the aorta rightward of the pulmonary artery. Accuracy of this schema for assigning ventriculo-arterial relationships is highly dependent on obtaining standard echocardiographic views, since some rotation from the standard plane can make a normally placed aorta appear to override a perimembranous VSD by more than 50%, thus leading to an incorrect anatomic diagnosis.
Using the more embryologically based approach of Van Praagh, one first describes the great arteries as normally related, transposed, or malposed. When there are normally related vessels, the aorta is posterior to the pulmonary artery, and the relationship between them can be noted as situs solitus (s)
when the aorta (aortic valve) is rightward of the pulmonary artery (pulmonary valve), or situs inversus (i) when the aorta is leftward of the pulmonary artery (Fig. 68.4). In transposition, the aorta is generally anterior to the pulmonary artery, and the relationship can be described as dextro (d) when the aorta is rightward, or levo (l



when the aorta (aortic valve) is rightward of the pulmonary artery (pulmonary valve), or situs inversus (i) when the aorta is leftward of the pulmonary artery (Fig. 68.4). In transposition, the aorta is generally anterior to the pulmonary artery, and the relationship can be described as dextro (d) when the aorta is rightward, or levo (l
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




