Carbon Dioxide Absorbers Save Gas and Moisture But Create the Potential for Mechanical Hazards, Chemical Soup, or A Thermal Disaster
Michael Axley MD, MS
Stephen T. Robinson MD
The purpose of anesthesia circuits is to provide an efficient configuration to deliver oxygen and anesthetic gases and vapors to the patient. If only fresh gas is going to be delivered, the required gas flows may need to be as high as two and one half times the patient’s minute ventilation (MV) to avoid rebreathing of carbon dioxide (CO2). A patient with an MV of 6 L/min would require a fresh gas flow of 15 L/min. Using a CO2 absorber, fresh gas flows of 500 ml/min or lower can be achieved.
BASIC MANAGEMENT OF ABSORBERS
Currently available CO2 absorbents consist of soda lime (sodium hydroxide lime), barium hydroxide lime, potassium-hydroxide-free lime, calcium hydroxide lime, and non-caustic lime. These absorbents all contain hydroxide bases as their active component. The main reactant in these absorbents (except for in barium hydroxide lime) is calcium hydroxide. Since it is a slow reactant, other constituents are needed to allow the conversion to occur at a sufficient rate in vivo.
Soda Lime Reaction:
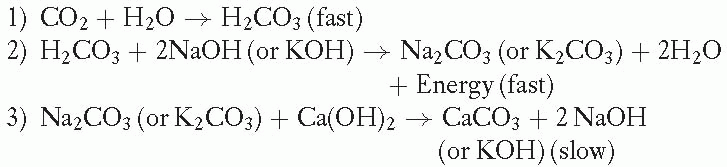
The CO2 absorbent is typically stored in two containers that operate in sequence on the expiratory limb of the anesthetic circuit. They lie distal to the ventilator relief valve and the adjusting pressure limiting (APL) valve. Hence, exhaled gas is preferentially routed to the scavenging system before it is cleared of CO2. The lower the fresh gas flow, the more rapid the consumption of absorbent.
Because the flow of expired gas is unidirectional, most of the reaction occurs in the proximal chamber. As the reactant is consumed, the pH changes and the indicator in the white granules turns blue. Generally, the first
chamber shows substantial change in color before the second chamber shows any change in color. The second chamber begins to change in color when the amount of available reactant becomes insufficient to eliminate fully the CO2 at lower flows. It is then necessary to change the reactant or to use higher fresh gas flows.
chamber shows substantial change in color before the second chamber shows any change in color. The second chamber begins to change in color when the amount of available reactant becomes insufficient to eliminate fully the CO2 at lower flows. It is then necessary to change the reactant or to use higher fresh gas flows.
Caustic water and residue can build up at the base of the absorber and along the distal tubing. To avoid inadvertent obstruction to gas flow, draining the fluid periodically and following routine maintenance recommendations, as provided by the anesthesia machine manufacturer, are necessary.
MECHANICAL COMPLICATIONS
Channeling is a condition that occurs when the gas flow through the absorbent is diverted through areas of low resistance, causing a ‘channel’ to be formed. Gas flowing through a channel does not undergo the reaction clearing CO2—hence, absorbents that form channels are less effective. More ominously, absorbent that is exhausted or in which channeling has occurred may permit rebreathing of CO2 through the circuit, with subsequent patient morbidity.
The issue of channeling is related to that of absorbent pellet size. Air flow through a mass of pellets varies inversely with pellet size; that is, larger pellets generate less resistance through the circuit. At the same time, large pellets expose less total surface area to air flow, decreasing the total surface area available to participate in the reaction that removes CO2. The most commonly available pellet size for CO2 absorbents is 4 to 8 mesh. “Mesh” indicates the number of openings per linear inch in a sieve used to measure the pellet particles.
Not all CO2 rebreathing is related to consumption of CO2 absorbent. Inspiratory and expiratory valves stuck in the open position can cause rebreathing. Kinked anesthesia hoses can also play a role.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



