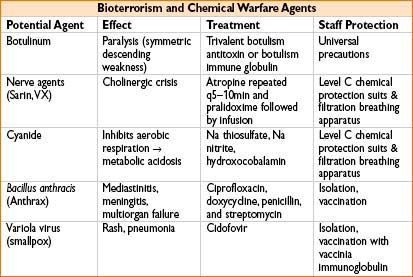
NASCIS Protocol for Steroid Therapy
• High-dose methylprednisolone is no longer recommended as standard of care for blunt ASCI. Multiple studies have suggested that the risks of high-dose steroid therapy outweigh benefits for ASCI.
• If used, must be initiated within 8 hrs of injury
• Methylprednisolone loading dose 30 mg/kg IV over 1 hr followed by:
• 5.4 mg/kg/hr IV infusion for next 23 hrs if started within 0–3 hrs of injury (NASCIS II: N Engl J Med 1990;322:1405–1411)
• 5.4 mg/kg/hr IV infusion for 47 hrs if started within 3–8 hrs of injury (NASCIS III: JAMA 1997;277:1597–1604)
• No evidence support the use of steroids in penetrating ASCI
• Unclear evidence in patients with documented complete thoracolumbar ASCI
• Must administer stress ulcer & hyperglycemia prophylaxis during steroid therapy
Neurogenic Shock
• Triad of hypotension, bradycardia, and hypothermia from functional sympathectomy (loss of vascular tone) and/or loss of cardiac inotropy/chronotropy secondary to high spinal cord injury
• More common in midthoracic injuries and higher
• In high cord injuries, loss of cardiac accelerator function & unopposed parasympathetic tone contribute to bradycardia (exacerbates impaired cardiac output)
• Consider anticholinergics/β-agonists to increase heart rate
• Consider α-agonists to restore peripheral vascular tone & improve venous return
Spinal Shock
• Disruption of all cord function caudal to spinal cord injury
• Causes flaccid weakness/lack of spinal arc reflexes at and below injury
Autonomic Hyperreflexia
• Most common in injuries above T6, occurs weeks to months after the acute injury
• Results from loss of descending inhibitory impulses + sympathetic system overactivity
• Stimulation below injury level (bladder distention, surgical stimulation) causes:
• Vasoconstriction/hypertension below injury
• Reflex bradycardia & dysrhythmias
• Vasodilation above injury
• Symptoms: Headaches, blurred vision, seizures, cerebral hemorrhage, pulmonary edema 2° to left heart failure, loss of consciousness, nasal congestion, cutaneous flushing
• Treatment:
• Remove stimulus
• Consider atropine for severe bradycardia
• Hypertension treated with direct vasodilators (nitroprusside/nitroglycerin), α-blockers (prazosin), ganglionic blockers (trimethephan)
• Consider using general anesthesia or spinal; epidural may not be as effective because of sacral sparing effect
Intraoperative Trauma Management

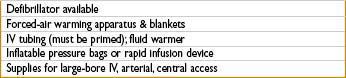
Goal: Optimize pt stability while not delaying surgical control of bleeding. Clear communication must be maintained despite chaotic environment.
Maintenance of Anesthesia
• Maintenance options may include a combination of hypnotic, analgesic, and neuromuscular blocking agents
• Normal doses of volatile agent may cause hypotension in unstable pts
• Nitrous oxide typically avoided due to potential pneumothorax/other air collections
• Trauma pts are at high risk of intraoperative recall (consider EEG monitoring)
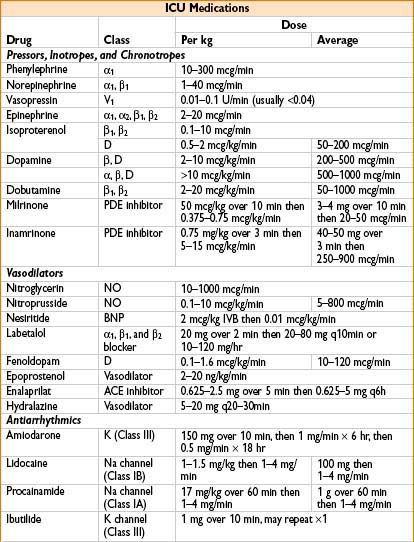
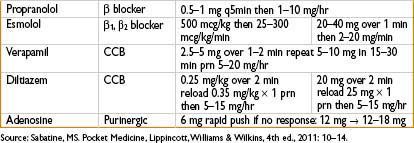
Management of Hypotension
• Ensure adequate cardiac preload (volume resuscitation)
• Vasopressors as necessary, consider categories of shock:
• Hypovolemic shock (hemorrhage)
• Cardiogenic shock (myocardial injury/existing heart disease)
• Obstructive shock (pericardial tamponade, tension pneumothorax, pulmonary emboli)
• Distributive shock
• Neurogenic shock (cord injury)
• SIRS/Sepsis
• Anaphylaxis
• Communicate all use of pressors/inotropes to surgical team
• Surgeons can often adjust clamp/pack placement, or organ manipulation to help if needed
Emergence/Transport
Preparation before transporting intubated/sedated patient to the ICU includes:
• Functioning bag valve mask + full oxygen tank
• Equipment for mask ventilation/reintubation
• Emergency drugs (phenylephrine, ephedrine, atropine, epinephrine, succinylcholine)
• Transport monitor (SpO2, ECG, blood pressure)
• Assistance to move the bed & secure elevators
Considerations during emergence:
• Severe hypertension may disrupt clots
• Airway may be edematous after volume resuscitation
• An “easy airway” at induction might be difficult after massive fluid administration
• Ensure help and equipment for rapid reintubation available
Fluid Resuscitation
Overall goal: Maintain perfusion to vital organs while surgeon controls hemorrhage

Obtain Adequate Vascular Access
• Peripheral IVs (14-gauge catheters—can deliver up to 500 mL/min)
• Peripheral Rapid Infusion Catheter (can deliver 850 mL/min)
• 9-Fr central venous “introducer” catheter (can deliver 1000 mL/min) (Note: Multiport central venous catheters deliver at far slower rates than 14 gauge IVs)
• Consider rapid infusion devices and/or pressure bags (must take care to avoid administration of IV air when giving fluids under pressure)
Fluid Resuscitation
• Advantage of crystalloid vs. colloid continues to be debated in trauma resuscitation. Albumin colloid solutions are contraindicated in head trauma patients
• Administer warm lactated Ringer’s while blood is obtained (hypothermia can cause failure of coagulation cascade)
• Avoid excessive administration of fluids (causes dilution of platelets/coagulation factors, reversal of compensatory vasoconstriction, breakage of clots due to rapid volume expansion)
Transfusion therapy (see Chapter 9, Fluids, Electrolytes, and Transfusion Therapy)
• Packed red blood cells (PRBCs)
• Uncrossmatched blood (type O)—use if pt has unstable hemorrhagic shock
• Type-specific blood—substitute for type O as soon as possible
• Transfusion rate depends on bleeding rate
• Goal is to maintain Hb above 7–9
• Fresh frozen plasma (FFP)
• For unstable, bleeding patients in or near hemorrhagic shock, consensus guidelines now recommend to use the 1:1:1 strategy: transfuse 1 unit of FFP for every unit of RBC given combined with platelet transfusion
• Consider transfusing 1 unit of FFP for every 1 unit of PRBCs (exact ratio controversial)—also use INR/PTT/clinical picture as guide
• ABO compatibility required, Rh compatibility not required
• Platelets
• Platelet count >50,000 generally desirable.
• Adverse effects of massive transfusion
• Calcium depletion 2° to citrate chelation
• Transfusion reaction (given cumulative risk of clerical errors)
• Hyperkalemia (secondary to hemolysis in stored blood)
• Transfusion-related immunomodulation (TRIM)
• Transfusion-related acute lung injury (TRALI)
• Volume overload/congestive heart failure (CHF)
Monitoring During Fluid Resuscitation
• Arterial systolic pressure waveform variation (with positive-pressure ventilation) is a useful marker of fluid responsiveness
• Urine output
• Serum markers of global tissue perfusion
(degree of acidosis, base deficit, lactate, central/mixed venous O2 saturation)
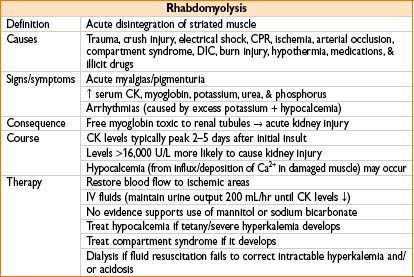
Nutrition
Enteral feeding—preferred over parenteral nutrition (promotes maintenance of intestinal tissue)
Total Parenteral Nutrition (TPN)
• Use only when adequate enteral feeding not possible
• Must be given centrally (due to ↑ osmolarity)
• Typical TPN formula: Carbohydrates 50–60%, proteins 15–25%, lipids 20–30%
Insulin therapy/frequent glucose monitoring required to avoid hyperglycemia → insulin can be added to TPN solution
• Monitor labs at least weekly: Electrolytes, aminotransferases, alkaline phosphatase, bilirubin, triglycerides, cholesterol, prealbumin, and transferrin
• Complications
→ Infection/sepsis, excessive CO2 production, hepatic steatosis, hyperglycemia, hyperlipidemia, impaired immune function, electrolyte derangements, muscle weakness
• Intraoperative management
→ Avoid abrupt cessation of TPN without replacing carbohydrate source (theoretical risk of hypoglycemia)

Glycemic Control in ICU Patients
• Older evidence suggested that insulin infusions for aggressive glucose maintenance (80–110) improve outcome
• Recent data suggest that aggressive glucose maintenance (81–108) increases risk of mortality compared with conventional glucose maintenance (<180)
→ Risk of tight glycemic control = hypoglycemia (appears to outweigh benefit)
• Intra-op glycemic control: Conventional glucose maintenance is safer than aggressive glucose maintenance
Burn Management
Pathophysiology
• Skin destruction → impairs heat regulation, fluid/electrolyte maintenance, microbial barrier
• Circulating mediators trigger systemic inflammatory response
→ Hypermetabolism, immunosuppression, & alteration in cell membrane permeability
→ Massive fluid shifts from vascular compartment to burned tissue
→ Edema occurs in burned tissue as well as in unaffected tissues
→ Fluid loss from intravascular space can cause hypovolemic shock
Initial Evaluation and Management
Depth of burn injury—1st, 2nd, & 3rd degree classification system replaced by:
• Partial-thickness burn: Destruction of epidermis & portion of dermis
(blanches with pressure & retains sensation of pain)
• Superficial partial thickness → confined to upper third of dermis
• Mid partial thickness → involves middle third of dermis
• Deep partial thickness → leaves only a portion of dermis viable
• Full-thickness burn: No dermis is viable
(will not blanche with pressure & is insensitive to pain)
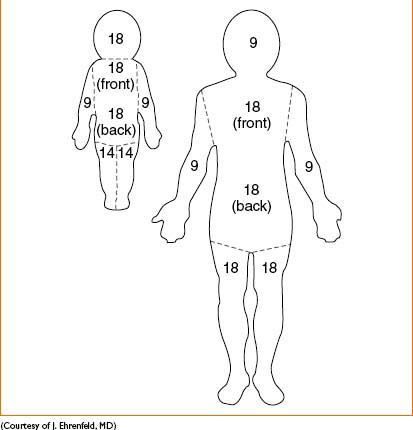
Total body surface area (TBSA; Fig. 15-1)
• “Rule of nines” estimates surface area burned in adult patients (see diagram below)
→ Head & each upper extremity represent = 9% TBSA
→ Anterior trunk, posterior trunk, & each lower extremity = 18% TBSA
→ Less accurate in children, due to different bodily proportions (see diagram below)
Airway and Respiratory Management
• Deliver maximal FiO2 during initial resuscitation of major burn victims
• Inspiration of hot gases
→ Can cause direct airway damage/obstruction from edema
• Tracheal intubation usually indicated in large burns → perform before airway edema renders this impossible
• Suspect possible inhalation injury when head/neck involved
(singed nasal hairs, swelling of nose, mouth, lips, throat; cough productive of soot)
Figure 15-1. Rule of nines.
• Chest wall expansion may be ↓ in major chest burns → consider emergency escharotomies
→ All inhalational injuries = potential “difficult airway” (due to cord edema); prep for surgical airway
Cardiovascular and Fluid Management
• Parkland formula: 4 mL of lactated Ringer’s per kg per % TBSA burn in initial 24 hrs
• Half of calculated fluid given during 1st 8 hrs postburn, remainder over next 16 hrs (e.g., 70 kg man with 60% TBSA burn needs: 4 × 70 × 60 = 16,800 mL; give 8400 mL of LR during 0–8 hrs after burn, 8400 mL during 8–24 hrs)
• Patient’s daily maintenance fluid should be given concurrently
• Cardiac output reduced immediately postburn (↓ circulating vol + direct myocardial depression)
• 3–5 days postburn, hypermetabolic state → ↑ cardiac output (2–3 × normal), ↓ SVR
INTRAOPERATIVE MANAGEMENT: GENERAL CONSIDERATIONS

Anesthesia for Burn Surgery

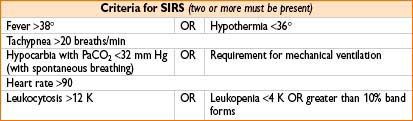
Sepsis/Systemic Inflammatory Response Syndrome (SIRS)
SIRS: Common causes include surgery, major trauma, pancreatitis/transfusion reaction

Acute Physiology and Chronic Health Evaluation (APACHE) II Score
• Most commonly used ICU illness scoring system: ↑ score = ↑ disease severity/risk of death
• Useful in initial evaluation of patients with severe sepsis
• Calculated based on: Temp, MAP, heart rate, respiratory rate, P(A-a)O2 or PaO2 (depending on FiO2), arterial pH, sodium, potassium, creatinine, hematocrit, WBC count, GCS score, HCO3, age, and chronic health status
• Several web-based calculators available (http://www.sfar.org/scores2/apache22.html)
Associated Problems in Sepsis
• Shock (from vasodilation, intravascular depletion +/- myocardial depression)
• Respiratory failure (from endothelial injury → alveolar capillary leak → impaired oxygenation)
• Acute lung injury (ALI)/Acute respiratory distress syndrome (ARDS)
• Renal failure/Metabolic acidosis
• Disseminated intravascular coagulopathy (DIC)
• Multiple organ dysfunction syndrome
Early Goal-Directed Therapy (EGDT) (N Engl J Med 2001;345:1368–1377)
• Strategy to ↓ mortality in pts with severe sepsis/septic shock by implementation of protocol (Overall goal: Optimize parameters that match oxygen demand & oxygen delivery)
• Specific goals include:
• CVP 8–12 mm Hg (maintained by crystalloid boluses)
• MAP 65–90 mm Hg (maintained by vasopressors or vasodilators)
• Urine output >0.5 mL/kg/hr
• Maintain central venous oxygen saturation >70%
Markers of Resuscitation in Sepsis
• Continuous or intermittent central or mixed venous oxygen saturation monitoring
Inadequate tissue perfusion → rise in oxygen extraction → low CVO2 or MVO2
• Trend of lactic acid level or base deficit
MANAGEMENT OF SEPSIS



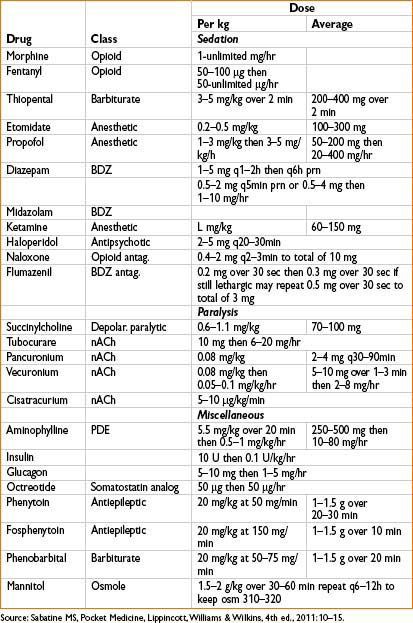
An approach to Sedation of ICU Patients
• Evaluate and treat pain (uncontrolled pain = risk factor for delirium) Anesthesiology 2006;104:21–26.
• Evaluate and address delirium
• Evaluate RASS (Richmond Agitation Sedation Scale) for level of sedation
If over-sedated, hold analgesics and/or sedatives until RASS is at goal, then reduce the amount of analgesics/sedatives from prior regimen
If under-sedated and receiving mechanical ventilation, titrate propofol until goal RASS is achieved (not to exceed 300 mcg/kg/min)
If under-sedated and NOT receiving mechanical ventilation, consider adding the following agents:
• Atypical anti-psychotics (e.g., haloperidol, quetiapine)
• Centrally acting cardiovascular medications (e.g., clonidine, propranolol) which have mild sedating effects
• Minimize the use of benzodiazepines (particularly in elderly or delirious patients)
If these agents are contraindicated or not effective, consider using dexmedetomidine 0.2–1 mcg/kg/hr. Omitting a loading dose reduces the incidence of bradycardia and hypotension.
Transfusion of Red Blood Cells (RBCs) in the Critically Ill Patient
In 2009 the Society of Critical Care Medicine published the results of an exhaustive analysis of trials. Key recommendations:
• RBCs should be transfused when a critically ill patient is suffering from hemorrhagic shock
• For hemodynamically stable patients transfusion of RBCs to maintain a hemoglobin ≥7 g/dL is as effective as transfusion to maintain a hemoglobin of ≥10 g/dL. A possible exception to this is acute myocardial ischemia. In the setting of stable cardiac disease, there is no clear benefit to maintaining hemoglobin ≥10 g/dL
• In the absence of ongoing hemorrhage, RBCs should be transfused as single units
• Transfusion should be avoided in ALI/ARDS patients unless absolutely necessary
• Transfusion decisions must be tailored to individual patients’ situations. Rigid protocols should be avoided
< div class='tao-gold-member'>









