9 Breathing and chronic pelvic pain Connections and rehabilitation features
Pelvic girdle pain: Respiratory connections
Varieties of breathing pattern disorder
Breathing rehabilitation assessment and interventions
Research on breathing as a pain intervention
Connective tissue manipulation
Trigger point deactivation and slow stretching
Myofascial release (myofascial induction)
Introduction
The lumbopelvic cylinder: Functional and structural connections
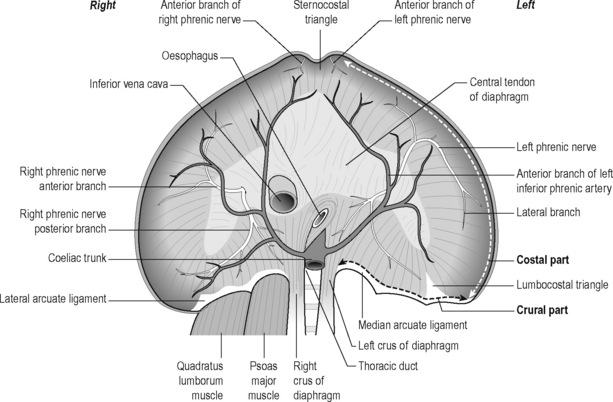
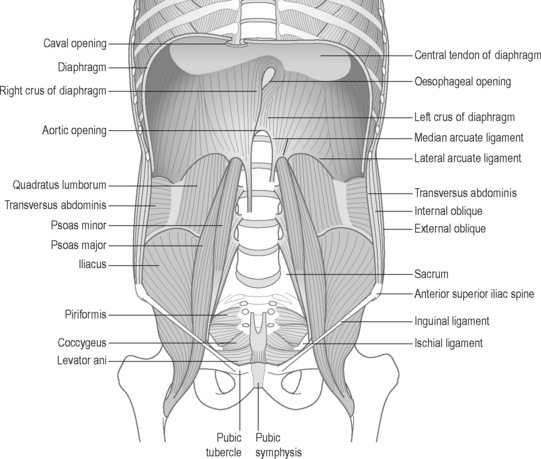
The pelvic floor and the respiratory diaphragm are structurally and functionally bound together by fascial and muscular connections (Figure 9.1). The abdominal canister has been described as a functional unit that involves the diaphragm, including its crura; psoas; obturator internus; deep abdominal wall and its associated fascial connections; deep fibres of multifidus; intercostals; quadratus lumborum; thoracolumbar vertebral column (T6–T12 and associated ribs, L1–L5) and osseus components of the pelvic girdle (Jones 2001, Gibbons 2001, Newell 2005, Lee et al. 2008). Gibbons (2001) has described the anatomical link between the diaphragm, psoas and the pelvic floor: ‘The diaphragm’s medial arcuate ligament is a tendinous arch in the fascia of the psoas major. Distally, the psoas fascia is continuous with the PF fascia, especially the pubococcygeus’. See Box 9.1 for detailed anatomy of the diaphragm.
Box 9.1 Anatomy of the diaphragm
The elliptical cylindroid-shaped diaphragm is a dome-shaped, musculotendinous structure with a non-contractile central tendon. Diaphragmatic fibres radiate peripherally to attach to all margins of the lower thorax, representing the inferior aspect of the pleural cavity, as well as a superior arch that covers the abdominal cavity (Pacia & Aldrich 1998). Its structures comprise both striated skeletal muscle and tendinous elements. When the diaphragm contracts, it increases the vertical, transverse and anteroposterior diameter of the internal thorax (Kapandji 1974).
The lumbar, costal and sternal muscular components (Schumpelick & Steinau 2000) (see Figures 9.1 and 9.2)
The muscular segments of the diaphragm originate from the entire circumference of the lower thoracic aperture: from the lumbar spine, ribs and sternum. There are three components, which are typically separated from each other by muscle-free gaps, the lumbar costal and sternal sections. These muscular diaphragmatic components insert at the central tendon, which is considered the central aponeurosis. In good health the diaphragm comprises type I, slow-twitch, fatigue-resistant muscle fibres as well as type II, fast-twitch, fatiguing muscle fibres. Fibre-type modifies in response to chronic obstructive pulmonary disease and to diaphragmatic inactivity (Anraku & Shargall 2009).
Lumbar (crural) part
1. Medial, which is tendinous in nature and lies in the fascia covering psoas major. Medially it is continuous with the corresponding medial crus and also attaches to the body of L1 or L2. Laterally it attaches to the transverse process of L1. The medial arcuate ligament is continuous medially with the lateral margin of the crus and is attached to the side of the body of the first or second lumbar vertebra. Laterally, it is fixed to the front of the transverse process of T12 and arches over the psoas muscle. Abnormal tensions in this ligament may irritate the psoas muscle, resulting in pain and spasm. Conversely psoas spasm may influence diaphragmatic mechanics (Burkill & Healy 2000, Carriero 2003, Carriere 2006).
3. The lateral arm, which is formed from a thick fascial covering that arches over the upper aspect of quadratus lumborum, to attach medially to the anterior aspect of the transverse process of L1, and laterally to the inferior margin of the 12th rib.
Carriero (2003) notes that the lateral arcuate ligament is a thickened band of fascia extending from the anterior aspect of the transverse process of the first lumbar vertebra to the lower margin of the 12th rib near its midpoint. It arches across the upper part of the quadratus lumborum muscle. Besides affecting respiratory excursion, dysfunction of the 12th rib may affect the lateral arcuate ligament, resulting in irritation of the iliohypogastric or ilioinguinal nerves that pass under it; ‘this may present as paresthesias or radiating pain over the anterior aspect of the thigh and groin with running activities’.
Costal part
Alternating with the dentations of the transverse abdominis muscle (Standring 2008), the costal part originates from the six caudal ribs, radiating into the central non-contractile tendon. In most cases, a triangle lacking muscle fibres, the lumbocostal triangle, exists between the lumbar and costal parts of the diaphragm, more commonly on the left side. In these weak areas, the gaps are usually closed only by means of pleura, peritoneum and fascia (i.e. fascia transversalis and fascia phrenicopleuralis).
Tendinous part
Schumpelick & Steinau (2000) note that the tendinous part (i.e. the central tendon) has ‘almost the shape of a cloverleaf (one anterior and two lateral leaves), with its largest expansion in the transverse plane’. The inferior vena cava, firmly anchored by connective tissue, passes through a foramen located to the right of the midline. The pericardium is also firmly attached to the cranial surface of the central tendon.
The left and right domes of the diaphragm arise lateral to the heart.
The right dome is commonly slightly higher than the left.
The location of the diaphragm is considered to be ‘variable’ (Schumpelick & Steinau 2000) depending on variables such as age, gender, posture and the extent of inhalation and exhalation, as well as on intestinal status. Any changes in the volume in the pleural or peritoneal cavity are likely to influence altered shape and position of the diaphragm.
Following full inhalation, the right dome of the diaphragm is situated close to the level of the cartilage–bone transition of the sixth rib, while the left dome is approximately one intercostal space lower (Tondury & Tillman 1998).
Innervation of the diaphragm
Schumpelick & Steinau (2000) observes that the peripheral parts of the diaphragm also receive motor innervation via the lower six intercostal nerves.
The phrenic nerve is located between the pericardium and mediastinal pleura.
Newell (2005) has further detailed the relationship between psoas and quadratus lumborum, with the diaphragm and thoracic structures, observing that the posterior edge of the diaphragm crosses the psoas muscles medially, forming the medial arcuate ligaments, and the quadratus lumborum muscles laterally, forming the lateral arcuate ligaments.
• The skeletal attachments of the lateral arcuate ligaments are the first lumbar transverse process and the midpoint of the 12th rib. The costal origins include the lower six ribs and costal cartilages, the fibres of the diaphragm interdigitating with those of transversus abdominis.
• The medial arcuate ligament is continuous medially with the lateral margin of the crus, and is attached to the side of the body of the first or second lumbar vertebra. Laterally, it is fixed to the front of the transverse process of T12, and arches over the psoas muscle. Abnormal tensions in this ligament may irritate psoas, resulting in pain and spasm. Conversely psoas spasm may influence diaphragmatic mechanics (Burkill & Healy 2000, Carriere 2006).
The retroperitoneal space
Lying between the posterior parietal peritoneum and the transversalis fascia is the retroperitoneal space, an anatomical region seldom discussed in relationship to CPP (Burkill & Healy 2000). This space houses (in whole or in part): the adrenal glands, kidneys, ureters, bladder, aorta, inferior vena cava, oesophagus (part), superior two-thirds of the rectum; as well as parts of the pancreas, duodenum and colon (Ryan et al. 2004). This area involves vital connections that intimately bind pelvic and thoracic structures. The anterior pararenal space extends superiorly to the dome of the diaphragm, and hence to the mediastinum. Inferiorly it communicates with the pelvis and below the inferior renal cone with the posterior pararenal space. The posterior pararenal opens inferiorly towards the pelvis but fuses superiorly with the posterior perirenal fascia the fascia of the quadratus lumborum (QL) and psoas muscles (Burkill & Healy 2000).
Grewar & McLean (2008) indicate that respiratory dysfunctions are commonly seen in patients with low back pain, pelvic floor dysfunction and poor posture. Additional evidence exists connecting diaphragmatic and breathing pattern disorders, with various forms of pelvic girdle dysfunction (including sacroiliac pain) (O’Sullivan et al. 2002, O’Sullivan & Beales 2007) as well as with CPP and associated symptoms, such as stress incontinence (Hodges et al. 2007). Similarly Carriere (2006) noted that disrupted function of either the diaphragm or the PFM may alter the normal mechanisms for regulating intra-abdominal pressure (IAP).
The presence of dysfunctional breathing patterns which influence pelvic function (McLaughlin 2009) and pelvic dysfunction which influences breathing patterns (Hodges et al. 2007) therefore suggests that rehabilitation of the thorax, pelvic girdle and pelvic floor will be enhanced by more normal physiological breathing patterns. This can be achieved through exercise, breathing retraining, postural reeducation, manual therapy and other means (Chaitow 2007, O’Sullivan & Beales 2007, McLaughlin 2009).
Interaction of CPP, pelvic girdle pain and breathing pattern disorders aetiological features
• Aetiologically, pregnancy or trauma may result in pelvic girdle problems and pain, via skeletal malalignment, such as separation of the symphysis pubis or sacroiliac dysfunction (Shuler & Gruen 1996).
• Additionally, the development of low back pain during pregnancy increases the odds of developing pelvic floor disorder complaints (Pool-Goudzwaard et al. 2004).
• The combined prevalence of lumbopelvic pain, incontinence and breathing disorders has suggest that pelvic floor dysfunction is related to altered breathing patterns or disorders of breathing (Smith et al 2006, 2007, O’Sullivan & Beales 2007).
• Hodges et al. (2007) observe that there is a clear connection between sacroiliac joint (SIJ) stability and respiratory and pelvic floor function, particularly in women. They suggest that if the PFM are dysfunctional, spinal support may be compromised, increasing obliquus externus activity, which in turn may alter PFM activity.
• It is suggested that any part of the structural unit, involving the respiratory diaphragm and the pelvic floor (‘pelvic diaphragm’), that fails to operate efficiently, will necessarily influence the function of other aspects of the complex.
Postural and breathing patterns as aetiological features
In a study involving 40 women with CPP, 20 received standard gynaecological attention, while the 20 women in the experimental group received the same attention, together with somatocognitive therapy, comprising postural, movement, gait and breathing assessment, re-education and rehabilitation. Haugstad et al. (2006a) observed that in the experimental group, women with CPP ‘typically’ displayed upper chest breathing patterns, with almost no movement of the thorax or the abdominal area. Haugstad et al. (2006a) were also able to confirm ‘a characteristic pattern of standing, sitting, and walking, as well as lack of coordination and irregular high costal respiration’. Of interest in relation to diaphragmatic function was their finding that: ‘the highest density, and the highest degree of elastic stiffness, [was] found in the iliopsoas muscles’.
Key (2010) suggests that clinicians should keep in mind: ‘the continuous, largely internal three dimensional myofascial web, providing a scaffold of tensile inner support and stability [ … ] contributing to a structural and functional bridge between the lower torso and legs’. Key also notes that: ‘This includes the obvious contractile elements for which there is accumulating evidence of deficient function in subjects with low back and/or pelvic pain – the transversus abdominis (Hodges & Richardson 1996, 1998, 1999), multifidus (Hides et al. 1996), the diaphragm and PFM’.
Impressions from clinical practice suggest attention should also be given to the obturators, iliacus, psoas, and all their related and interconnecting fascial sheaths. Sound activity within this myofascial ‘inner stocking’ sustains many functional roles: providing deep anterior support to the lower half of the spinal column; with the spinal intrinsic muscles it contributes to lumbopelvic control (Hodges 2004); while also contributing to the generation of IAP (Cresswell et al. 1994), continence and respiration (Figure 9.2).
The lack of normal diaphragmatic movement in individuals with breathing pattern disorders (BPD) deprives the viscera and abdominal cavity of rhythmic stimulation (internal ‘massage’) which may be important for maintaining normal pelvic circulation. Pelvic pain and congestion have been correlated with chronic muscle tension, chronic hypoxia, as well as accumulation of metabolites such as lactic acid and potassium (Kuligowska et al. 2005).
Jones (2001) has summarized the integrated structural and functional thoracopelvic unit as follows:
Pelvic girdle pain: Respiratory connections
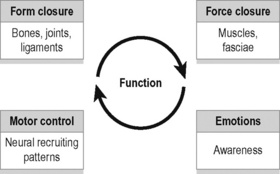
As discussed in Chapter 2, stabilization of the SIJs is enhanced by a combination of self-bracing and self-locking mechanisms, which have colloquially been described as ‘form closure’ (Vleeming et al. 1990a) and ‘force closure’ (Snijders et al. 1997, Hu et al. 2010) (Figure 9.3).
Cusi (2010) has suggested that shear is prevented by a combination of the specific anatomical features (form closure) and the compression generated by muscles and ligaments (force closure) that can accommodate to specific loading situations. Force closure has been defined as the effect of changing joint reaction forces generated by tension in ligaments, fasciae, and muscles and ground reaction force (Vleeming et al. 1990a, 1990b).
A significant part of this process involves increases in muscular, ligamentous and fascial stiffness, including that of the thoracolumbar fascia, and the multifidus and transversus abnominis, i.e. the major local stabilizers of the lumbar spine and the pelvis (Mens et al. 2001).
Additionally, and important to this discussion, using cadaveric studies the PFM have been shown to be capable of enhancing stiffness in the lumbar-pelvic region of women (Pool-Goudzwaard et al. 2004).
By performing biomechanical analysis of SIJ stability, Pel et al. (2008) have demonstrated that the training of transversus abdominis and the PFM helps to relieve SIJ related pelvic pain, via reduction of vertical shear forces. In rehabilitation of sacroiliac dysfunction, related to force closure, Cusi (2010) notes that a successful exercise programme needs to be specific, targeted and progressive. The initial demands of such a programme require the individual to develop the ability to recruit transversus abdominis, deep multifidus and the muscles of the pelvic floor.
Hodges et al. (2001) have demonstrated that, after approximately 60 seconds of over-breathing (hyperventilation), the postural (tonic) and phasic functions of both the diaphragm and transversus abdominis are reduced or absent, with major implications for spinal and sacroiliac stability. As major hip flexors the psoas muscles have the potential to influence pelvic girdle position and function. They should therefore attract therapeutic attention (along with the accessory breathing muscles) in any attempt to rehabilitate respiratory or pelvic function.
Gut connections to CPP and to respiration
Various studies of pelvic pain patients have shown irritable bowel syndrome (IBS) to be a common co-morbid condition (Zondervan et al. 1999, Whitehead et al. 2002). IBS, defined as pain more than once a month, associated with bloating and altered bowel habit (Moore & Kennedy 2000), is common in women with CPP. For example in one study, among 798 women referred to a gynaecology clinic, the incidence of IBS was 37%, compared to 28% among women attending ENT or dermatology clinics. Among those with chronic pain symptoms (including dyspareunia or dysmenorrhoea), the incidence was 50% (Prior et al. 1989).
Ford et al. (1995) have reported on the high incidence of increased colonic tone and dysfunction in hyperventilating individuals. Hypocapnic hyperventilation (low CO2 blood levels) produces an increase in colonic tone and phasic contractility in the transverse and sigmoid regions. These findings are consistent with either inhibition of sympathetic innervation to the colon, or the direct effects of hypocapnia on colonic smooth muscle contractility, or both.
It has also been observed – based on rectal and anal sphincter recordings – that during defecation the respiratory diaphragm and abdominal wall contract together, which results in an increase in IAP and rectal pressure (Olsen & Rao 2001).
Additionally pelvic floor contraction during exhalation allows for synergy between the pelvic and respiratory diaphragms (Prather et al. 2009), suggesting that when normal, respiratory function and the pelvic floor can be seen to synchronize intimately.
In the study by Prior et al. (1989), the authors did not seek to address whether IBS was the cause of the pelvic pain, but in a similar study, patients with symptoms of IBS were found to be less likely to receive a positive gynaecological diagnosis, and more likely to be still in pain, one year later, than patients without IBS symptoms (Whitehead et al. 2002). Rosenbaum & Owens (2008) note that gastroenterological conditions, such as coeliac disease and IBS, affect sexual function/comfort (Fass et al. 1998).
The anatomical location and innervation of both bladder and colon mean that they share similar vital functions, so that malfunction of one organ may result in a functional disturbance in the other. Furthermore the concepts of organ cross-talk, and organ cross-sensitization, between the bladder and the colon are important in the understanding of complex CPP syndromes (Watier 2009).
An integrated system
The concept of an integrated continence system (Grewar & McLean 2008) allows some coherence to be identified in apparently random presence of pain and dysfunction, in the pelvic region. Grewar & McLean suggest that the foundational mechanisms that support continence are relatively impervious to manual therapy when dysfunctional. However, there are also ‘external’ features that exert influence over these structural components – which are potentially modifiable.
• Motor control factors – including postural and movement dysfunction, BPD, pelvic floor dysfunction and low back and pelvic girdle dysfunction;
• Musculoskeletal features – including altered muscle strength, length and range of motion;
• Behavioural factors – such as physical inactivity, psychosocial issues, abnormal IAP and dysfunctional bowel and bladder habits.
There is evidence that respiration also has an influence on motor control (Butler 2000, Chaitow 2004) – emphasizing its importance amongst those factors to be considered in rehabilitation of continence dysfunction.
Varieties of breathing pattern disorder
Courtney et al. (2008) and Courtney & Greenwood (2009) suggest a distinction can be made between those BPD that appear to have a predominately biomechanical nature – where the patient may have a ‘perception of inappropriate, or restricted, breathing’, as distinguished from BPDs where a chemoreceptor aetiology may exist, for example linked to reported sensations such as there being a ‘lack of air’. Courtney et al. (2008) note that the sensory quality of ‘air hunger’ or ‘urge to breathe’ is most strongly linked to changes in blood gases, such as CO2, or changes in the respiratory drive deriving from central and peripheral afferent input. These sensations may be distinguishable from breathing sensations related to the effort of breathing, which are biomechanical in nature (Simon et al. 1989, Banzett et al. 1990, Lansing 2000, Chaitow et al. 2002).
Questionnaires exist for assessment of these BPD variations, with the Nijmegen Questionnaire (NQ) (van Dixhoorn & Duivenvoorden 1985) having greater relevance for hyperventilation, and the Self-Evaluation Breathing Questionnaire (SEBQ) (Courtney et al. 2009) discriminating between the chemoreceptor and the biomechanical variations of BPD (see Appendix).
Irrespective of the major aetiological features (see above and listed below in Box 9.2), chronic BPD results in altered function and, in time, structure of accessory and obligatory respiratory muscles. It is suggested that these should attract therapeutic attention in any attempt to normalize breathing, or the distant effects of BPD, on pelvic function (Chaitow 2004).
Box 9.2 Aetiological features in BPD
• Acidosis: Hyperventilation may represent a homeostatic response to acidosis. Chaulier et al. (2007) note that acidosis may result from iatrogenic sources, major hypoxaemia, cardiovascular collapse or sepsis.
• Atmosphere/altitude: ‘During expeditions … mountaineers have extremely low values of arterial oxygen saturation (SaO2), similar to those of patients with severe respiratory failure’. Hyperventilation would be the physiological response to this (Botella De Maglia et al. 2008). Altitude implications are not confined to mountaineers. Travellers to, for example, Johannesburg, Mexico City or Denver, would find themselves at altitude and potentially hyperventilating for some days, or weeks, before acclimatizing.
• Allergies/intolerances: Haahtela et al. (2009) report that airway inflammation commonly affects swimmers, ice hockey players, and cross-country skiers, which suggests multifactorial features in which both allergic and irritant mechanisms play a role in resultant over-breathing.
• Diabetic ketoacidosis (DKA): Patients with DKA generally present with classic clinical findings of hyperventilation, altered mental status, weakness, dehydration, vomiting and polyuria (Bernardon et al. 2009; see also Kitabchi et al. 2006).
• Hormonal – progesterone, oestradiol: Slatkovska et al. (2006) demonstrated that phasic menstrual cycle changes in PaCO2 may be partially due to stimulatory effects of progesterone and oestradiol on ventilatory drive. See also Damas-Mora et al. (1980).
• Pregnancy: Jensen et al. (2008) suggest that hyperventilation and attendant hypocapnia/alkalosis during pregnancy result from an interaction of pregnancy-induced changes in central chemoreflex drives to breathe and wakefulness, acid–base balance, metabolic rate and cerebral blood flow.
• Pseudo-asthma: A high proportion of individuals diagnosed as asthmatics have been shown to in fact be hyperventilators.
• Sleep disorders: There is an direct temporal, and possibly a etiological, connection, between sleep disorders and overbreathing, including sleep apnoea and cardiorespiratory fitness (Vanhecke et al. 2008).
Breathing pattern disorders – The postural connection
Carriere (2006) has reported that respiratory dysfunction is commonly observed in patients with low back pain and pelvic floor dysfunction.
Key et al. (2007) have observed and catalogued a number of variations within the patterns of compensation/adaptation associated with chronic postural realignment involved in crossed-syndromes commonly associated with pelvic deviation.
In Figure 9.4A the major features include:
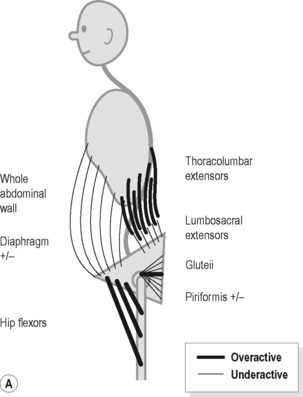
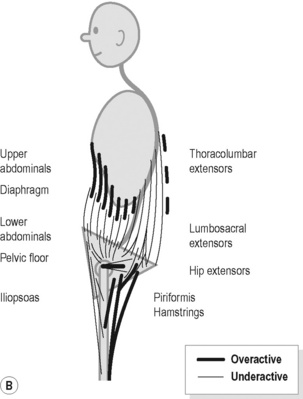
Figure 9.4 • Schematic views of (A) posterior (B) anterior pelvic crossed syndrome.
Reproduced from Key (2010) J. Bodyw. Mov. Ther. M. 14, 299–301.
In Figure 9.4B the major features include:
Examples
1. Haugstad et al. (2006a) evaluated 60 women with CPP, compared to healthy controls. They reported that in the standing posture, the area of support was minimal, with the feet being posed close together, the pelvic area pushed forward, and the shoulders and upper parts of the back pulled backwards. Compare this description with Figure 9.4B. In addition they identified a common pattern of high costal respiration with almost no movement in the thorax or in the abdominal area.
2. Psoas involvement has been identified in men with CPP. In a case-control series Hetrick et al (2003) noted that: ‘controls and patients with pain showed a significant difference in muscle spasm, increased muscle tone, pain with internal transrectal palpation of the pelvic muscles, and increased tension and pain with palpation of the levator ani and coccygeus muscles (P <0.001), as well as significantly greater pain and tension with palpation of the psoas muscles and groin’.
Repercussions of breathing pattern disorders
BPD has been shown to potentially have multiple, body-wide, influences which are summarized below.
Nixon & Andrews (1996) vividly summarize a common situation applying to the individual with BPD tendencies: ‘Muscular aching at low levels of effort; restlessness and heightened sympathetic activity; increased neuronal sensitivity; and, constriction of smooth muscle tubes (e.g. the vascular, respiratory and gastrointestinal) can accompany the basic symptom of inability to make and sustain normal levels of effort’.
Breathing pattern disorders (with hyperventilation as the extreme of this) may influence health by:
• Altering blood pH, creating respiratory alkalosis (Pryor & Prasad 2002, Celotto et al. 2008);
• Inducing increased sympathetic arousal, altering neuronal function – including motor control (Dempsey et al. 2002, Brotto et al. 2009);
• Encouraging a sense of apprehension, anxiety, affecting balance, muscle tone and motor control (Rhudy & Meagher 2000, Balaban & Thayer 2001, Van Dieën et al. 2003);
• Depleting Ca and Mg ions, enhancing sensitization, encouraging reduced pain threshold and the evolution of myofascial trigger points (Gardner 1996, Cimino et al. 2000, Schleifer et al. 2002, Simons et al. 1999);
• Triggering smooth muscle cell constriction, leading to vasoconstriction and/or spasm – including colon spasm (Ford et al. 1995, Yokoyama et al. 2008, Debreczeni et al. 2009) or pseudo-angina (Evans et al. 1980, Wilke et al. 1999);
• Reducing oxygen release to cells, tissues, brain (Bohr effect) so encouraging ischaemia, fatigue, pain and the evolution of myofascial trigger points (Freeman & Nixon 1985, Suwa 1995);
• Creating biomechanical overuse stresses and compromising core stability and posture (Lewit 1980, 1999, Haugstad et al. 2006b, Hodges et al. 2007).
BPD and hyperventilation: Physical features – Implications for rehabilitation
Deep and rapid breathing (hyperpnoea) results in progressive muscular fatigue and increasing sensations of distress, to the point of breathlessness. For example, Renggli et al. (2008) report that during normocapnic hyperpnoea (involving partial rebreathing of CO2), contractile fatigue of the diaphragm and abdominal muscles develops, long before task failure, triggering an increased recruitment of rib cage muscles. Since the diaphragm and abdominal muscles are key features of low back and pelvic stability, the implications for core instability of chronic, habitual, overbreathing – where normocapnic hyperpnoea would be unlikely – are clear. Respiratory alkalosis, and its numerous effects as described earlier in this chapter, would then accompany reduced pelvic and low back stability.
• The implication is that methods to help avoidance of hyperpnoea should be a feature of breathing retraining.
Hudson et al. (2007) observe that human scalenes are obligatory inspiratory muscles that have a greater mechanical advantage than sternocleidomastoid (SCM) muscles, which are accessory respiratory muscles. They found that irrespective of respiratory tasks these muscles are recruited in the order of their mechanical advantages – with scalenes starting to operate earlier than SCM, involving what they term to be an ‘efficient, fail-safe, system of neural control’.
• The implication in breathing rehabilitation is to ensure that these muscles receive focused attention as to their functionality.
Schleifer et al. (2002) recapitulate the known effects of overbreathing which they have identified as occurring in stress-related work settings:
• The implications suggest that these changes: ‘provide a unique rationale for coping with job stress and musculoskeletal discomfort through breathing training, light physical exercise, and rest breaks’.
Masubuchi et al. (2001) used fine-wire electrodes inserted into muscles, and high-resolution ultrasound, to identify the activity of three muscle groups, in response to various respiratory and postural manoeuvres. They concluded that the scalenes are the most active, and trapezius the least active, cervical accessory inspiratory muscles, while SCM is intermediate.
• This confirms what has long been suspected by observation and palpation – that the scalenes are the most important respiratory muscle group lying superior to the thorax.
Scalene dysfunction and the presence of trigger points (‘functional pathology’) were identified in excess of 50% of individuals, in a series of 46 hospitalized patients who demonstrated paradoxical patterns of respiration. A combination of Muscle Energy Technique (‘post-isometric relaxation’) and self-stretching of the scalenes, was used during rehabilitation (Pleidelová et al. 2002).
• The implication is that these key respiratory muscles require focused attention via palpation and appropriate therapeutic interventions, as part of breathing rehabilitation.
Renggli et al. (2008) showed (see above) that the progressive fatigue of the diaphragm and abdominal muscles, during overbreathing, results in recruitment of the muscles of the rib cage (intercostals).
Han et al. (1993) described the action, and interaction, of these rib cage muscles, during ventilation, noting that the parasternal intercostal muscles, act in concert with the scalenes to expand the upper rib cage, and/or to prevent it from being drawn inward by the action of the diaphragm, during quiet breathing. The respiratory activity of the external intercostals however appear to constitute a reserve system, only to be recruited when increased expansion of the rib cage is required.
• The implications of this information point to the need for attention to the often-neglected intercostal muscles, during breathing rehabilitation.
Earlier in this chapter the relationship between the psoas and quadratus lumborum muscles, and the retroperineal space, the pelvic floor, the pelvic girdle and respiratory function have been summarized (see Burkill & Healy 2000, Hetrick et al. 2003, Haugstad et al. 2006a, Key et al. 2007, Lee et al. 2008).
Viscerosomatic effects
Prather et al. (2009) expand on these relationships in a review of the anatomy, evaluation and treatment of musculoskeletal pelvic floor pain in women. They note that persistent muscle contraction of the pelvic floor, related to noxious visceral stimulation, such as that deriving from endometriosis or IBS, can lead to splinting and pain, with reduction of normal PFM function. Specifically, they report that viscerosomatic reflex activity may be responsible for increased resting tone of the pelvic floor with reduced ability to fully relax the muscle group as a whole. As a result, they suggest, adaptation occurs via recruitment of global muscles in the region – e.g. psoas and iliacus – leading to symptoms such as posterior pelvic and low back pain.
Tu et al. (2008) compared the biomechanical features of the pelvic girdle, as well as the associated muscles in 20 CPP patients and 20 normal controls. Among their findings – relevant to this chapter – are the following:
1. Several tests of pelvic girdle instability were more common in CPP cases than in controls (asymmetric iliac crest and pubic symphysis heights and positive posterior pelvic provocation testing).
2. In addition, patients with CPP were more tender on palpation of the left oblique, right and left rectus and right psoas (P > 0.05). Previous evidence, described above, suggests that such changes would be likely to impair respiration; however, this feature was not a part of Tu et al.’s study.
• Optimal rehabilitation of breathing function, requires appropriate attention to both psoas and QL status, as well as key accessory and obligatory respiratory muscles. Additionally, optimal rehabilitation of the pelvic floor requires attention to respiration and the multiple structures that influence it.