KEY POINTS
1. Both anemia and transfusion carry significant risks. Balancing these risks is the key to appropriate blood transfusion decisions.
2. Despite published guidelines about transfusion, clinical transfusion practice still varies substantially among clinicians and centers and often falls outside recommended guidelines.
3. In addition to well-established acute immunologic and infection transmission complications, RBC transfusion in cardiac surgical patients has more recently been associated with increased mortality and infectious complications.
4. Transfusion-related acute lung injury (TRALI) is the most frequent cause of mortality complicating blood transfusion. Among blood components, fresh frozen plasma (FFP) may carry the highest risk for TRALI.
5. RBC storage duration has been associated with increased morbidity and mortality in cardiac surgery.
6. One retrospective study associated platelet transfusion with increased morbidity and mortality in cardiac surgery, but several other studies did not.
7. FFP transfusion alone has been associated with increased risk for perioperative mortality, infection, multiorgan failure, and acute respiratory distress syndrome.

“BLOOD TRANSFUSION IS LIKE MARRIAGE: it should not be entered upon lightly, unadvisedly or wantonly or more often than is absolutely necessary.”
—R. Beale R [1]
1
I. Background
Competing risks form the core of perioperative transfusion. We are faced with tangible risks of patient complications from low hemoglobin values, yet we face different but equally real risks when we expose patients to red blood cell (RBC) transfusion. The risks of both anemia and transfusion vary with patient comorbidities, degree of and ability to tolerate anemia, and surgical procedure. In the dynamic milieu of the operating rooms, clinicians caring for cardiovascular surgical patients face challenging transfusion decisions daily. There are no measures that can definitively direct RBC transfusion decisions, rather clinical judgment using the balance of perceived risk versus benefit guides individual transfusion decisions. Given this background, the substantial variability of RBC transfusion even within a single institution becomes understandable [2,3].
2
A. Variability in practice patterns often serves as the impetus for evidence-based guidelines that are intended to reconcile disparate evidence. Recent work highlights the low level of practitioner adoption of published transfusion practice guidelines. Although a number of factors contributed to low physician adoption, the authors principally attributed it to the low level of evidence provided to support guideline recommendations [4]. Others have also reported limited evidence basis for use of RBC transfusion [5].
B. The reports on complications of RBC transfusion have increased scrutiny of transfusion practices in the last decade; however, economics and anticipated changes in population demographics have also driven change. A population-based cross-sectional study projected a growing gap between future demand and donation of blood with substantial increases in blood demand related to the changes in population demographics [6]. A recent editorial noted that 55% to 60% of the blood products transfused in the United States are given to the patients over 65 yrs old, and projected that this population will increase by 36% in the next 10 yrs, whereas the principal blood donor population will only increase by 5% over the same period. These demographic changes will influence medical practices and surgical procedures while increasing demand for blood products [7].
II. Red Blood Cell Transfusion and Adverse Outcomes
RBC units contain red cells separated from whole blood by centrifugation or by apheresis [8] (Table 18.1). Isbister and colleagues reported on the abundance of data supporting morbidity risk associated with transfusion, yet noted transfusion remains “ingrained” in current medical practice almost as a “default” position [9]. Furthermore, establishing causation between RBC transfusion and morbidity is difficult because of the potential for confounding in cohort study designs. Some argue that the etiology of poor outcomes relates to the “sicker” patient who receives a transfusion rather than the RBC product itself. Although it is difficult to discern whether morbidity is patient or transfusion related, either way RBC transfusion remains a significant risk factor for poor outcome [9].
Table 18.1 Fast facts: red blood cells from American Red Cross compendium8
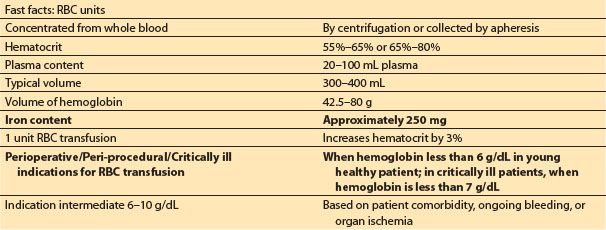
3
A. Morbidity and Mortality Risk
1. RBC transfusion has been associated with a number of immunologic complications such as hemolytic, anaphylactoid and febrile transfusion reactions, graft-versus-host disease, and transfusion of viral and bacterial contaminants [8]. RBC transfusion has been associated with an increased risk for a number of complications following surgery. In an isolated coronary artery bypass grafting (CABG) population of over 11,000 patients, RBC transfusion was associated with a dose-dependent increased risk for morbidity: More postoperative cardiac complications, prolonged postoperative ventilator support, more infectious complications, renal failure, and higher in-hospital mortality (Fig 18.1). Following risk adjustment for known confounders, RBC transfusion remained a significant risk factor for poor clinical outcomes. The effect of transfusion on morbidity was dose-dependent, that is, more RBC units transfused incrementally increased risk (Fig. 18.2). A number of factors were related to patients’ need for transfusion: Demographics such as older age, history of peripheral vascular disease and chronic obstructive pulmonary disease, clinical presentation such as emergency surgery, and surgical factors such as reoperation and prolonged aortic clamp time [10].
Figure 18.1 Forest plot displays the unadjusted and adjusted odds ratios and their 95% confidence limits for seven postoperative morbid outcomes when comparing patients undergoing cardiac surgery who did and did not receive an RBC transfusion. (Reproduced with permission from Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.)
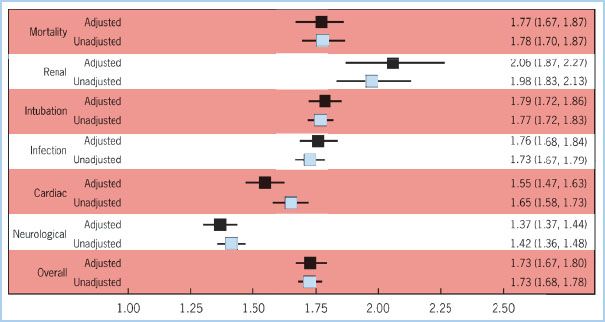
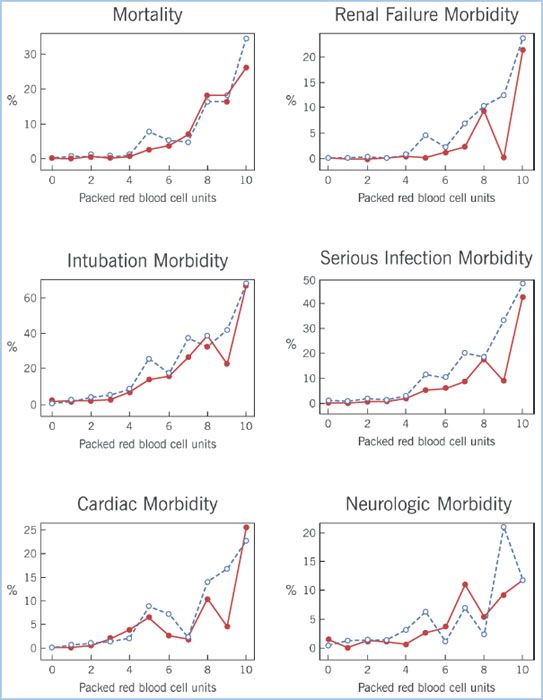
Figure 18.2 Panel of six morbidity outcomes displays the dose–response relationship between increasing number of RBC units transfused and risk for postoperative morbidity outcomes. The dashed lines represent the patient who received only RBC transfusion; the solid lines represent those patients who received platelet transfusion as well. (Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.)
2. Kudivalli and colleagues examined the effect of RBC transfusion on 30-day and 1-yr mortality in cardiac surgical patients. Risk-adjusted mortality at 30 days and 1 yr was significantly higher for patients who were transfused. Risk factors for RBC transfusion were lower hemoglobin, lower body mass index, female gender, renal dysfunction, reoperation and use of cardiopulmonary bypass [11]. An investigation of long-term survival in over 10,000 isolated CABG patients reported a risk-adjusted reduction in survival both early and late to 10 yrs for patients who received perioperative RBCs. This relationship was dose dependent and was sustained after adjustment for confounding variables known to increase mortality risk following isolated CABG [12].
3. A recent investigation examined over 9,000 patients who underwent cardiac surgical procedures and who were and were not exposed to 1 to 2 unit RBC transfusion. Patients who received RBCs had a 16% increased hazard for reduced survival 6 mos following the cardiac surgery. The authors emphasized the decision to transfuse even these small volumes of RBCs placed patients at significant risk for mortality [13]. Others have also reported reduced survival in cardiac surgical patients receiving RBC transfusions [14].
4. Murphy and colleagues examined outcomes after RBC transfusion in over 8,500 cardiac surgical patients. There was a strong association between transfusion and postoperative infectious complications, early and late mortality, length of stay, and overall hospital costs. The authors reported a significant risk for composite ischemic outcome noting this was somewhat counterintuitive as transfusion is intended to restore tissue oxygenation [15].
5. Banbury and colleagues identified risk factors for the occurrence of postoperative infectious complications following cardiac surgery in a population of over 15,000 patients in which over 50% of patients were transfused. There was a dose-dependent effect of increasing RBC transfusion and increased prevalence of postoperative septicemia/bacteremia and superficial and deep sternal wound infections. These authors appropriately noted that transfusion occurred in patients who were the sickest; yet, even following risk adjustment, transfusion contributed to postoperative infectious risk [16].
6. Transfusion of RBCs plays a role in augmenting the inflammatory response to cardiac surgery. Development of atrial fibrillation is a common and costly complication following cardiac surgery that links partially an inflammatory mechanism. In a population of 5,841 cardiac surgical patients, transfusion of RBCs increased the prevalence of postoperative atrial fibrillation. The analysis was risk-adjusted for traditional risk factors associated with development of atrial fibrillation such as demographics, history of atrial fibrillation, preoperative medications, and perioperative variables. RBC transfusion increased risk for postoperative atrial fibrillation in both on- and off-cardiopulmonary bypass procedures [17].
7. A recent publication by Tuinman and colleagues related complications of transfusion during cardiac surgery to activation of pulmonary inflammation and coagulation [18].
4
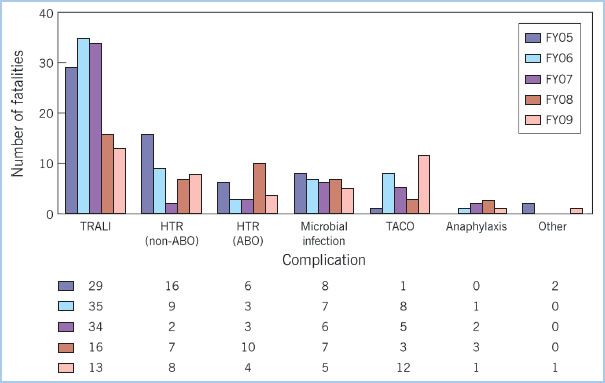
8. The Food and Drug Administration’s (FDA) summary of fatalities related to transfusion for fiscal year 2009 listed transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) among the more common complications associated with transfusion (Fig. 18.3). TRALI has consistently been one of the most common causes of death associated with transfusion [19]. TRALI, which is primarily a clinical diagnosis, can vary from mild to severe. It is defined by presence of hypoxemia and specific X-ray findings that occur within a certain time following transfusion. The purported mechanism for TRALI involves activation of neutrophils with endothelial injury and resultant pulmonary edema [20–22].
Figure 18.3 A report from the Food and Drug Administration illustrates the transfusion-related fatalities by complication for fiscal years 2005–2009. For each year TRALI represents the most frequently reported cause of transfusion-related mortality [19]. TRALI, transfusion-related acute lung injury; HTR (non-ABO), hemolytic transfusion reactions unrelated to ABO incompatibility; HTR (ABO), hemolytic transfusion reactions related to ABO incompatibility; TACO, transfusion-associated circulatory overload; FY05, Fiscal Year 2005 (similarly for other FYs).
9. A recent investigation noted that TRALI, a diagnosis of exclusion, can be difficult to diagnose in patients undergoing cardiac surgery. The authors reported greater pulmonary morbidity in the postoperative period for patients transfused with RBCs and FFP; this pulmonary morbidity may have been related to TRALI, TACO, or both. Nevertheless, transfusion of either RBCs or FFP was independently associated with pulmonary morbidity in the postoperative period [23].
10. Stokes and colleagues recently examined the influence of bleeding-related complications, use of blood products, and costs in a population of inpatient surgical services. They recognized that inadequate surgical hemostasis leads to bleeding complications as well as to transfusion. The authors were able to rank incremental cost per hospitalization associated with bleeding-related complications and adjusted for covariates. Their findings support further need for implementation of blood conservation strategies [24].
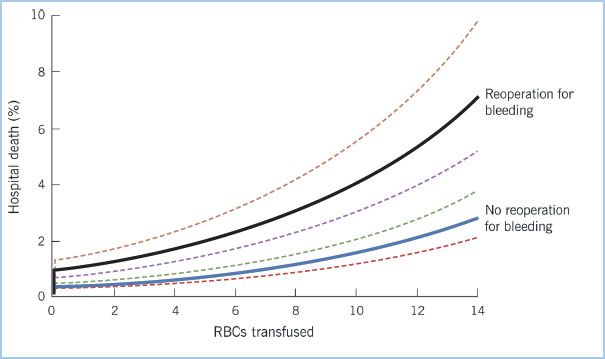
11. Bleeding complications and the associated need for reoperation in cardiac surgery are associated with increased morbidity. Recent work attempted to delineate whether increased morbidity risk for reoperation was related to the reoperation, blood transfusion, or both. The patients who underwent reoperation had greater subsequent morbidity, increased resource utilization, and higher in-hospital mortality even after risk adjustment [25] (Fig. 18.4).
Figure 18.4 Predicted probability of operative mortality stratified on reoperation for bleeding and RBC transfusion. RBC transfusion and reoperation for bleeding were associated with increased mortality. (Reproduced with permission from Vivacqua A, Koch CG, Yousuf AM, et al. Morbidity of bleeding after cardiac surgery: Is it blood transfusion, reoperation for bleeding, or both? Ann Thorac Surg. 2011;91:1780–1790.)
12. A recent investigation in non-cardiac thoracic surgical patients reported worse postoperative outcomes in patients who received 1 to 2 unit RBC transfusions. The negative effect of transfusion was dose dependent and was associated with increased morbidity and resource utilization. The authors urged clinicians to be cautious in transfusing patients for mild degrees of anemia [26].
13. Grimshaw and colleagues emphasize that the use of RBCs has not been scrutinized using current methods for assessing efficacy and safety, and that mechanisms for the observed complications are not well understood. They suggest that RBC transfusion-associated inflammation and thrombosis may be related to RBC storage [27].
B. Red Blood Cell Storage Duration
Changes in RBC product with increasing storage are well described and include alterations in RBC structural shape and in biochemical properties. Implications of these time-dependent changes may contribute to adverse clinical outcomes associated with RBC transfusion. However, clinical and experimental animal studies have reported inconsistent findings [28–30].
1. A group of investigators examined age of RBC products at the time of transfusion to gain an understanding of the age of RBC products used. Approximately one-third of RBC units transfused were stored longer than 3 wks storage duration, and for blood groups AB negative and A negative, more than 60% of total number of units transfused were older than 3 wks storage duration [31].
2. Survival of red cells following transfusion is an important variable as it relates to the ability of red cells to perform their intended use. The FDA currently allows donor RBCs stored in ADSOL to have a shelf life of 42 days. The set time limit relates to a requirement for 75% of RBCs to remain in circulation 24 hrs after transfusion, without regard to their functional state. With fresh blood, RBC survival is approximately 90% 24 hrs post-transfusion. As storage duration increases, 24-hr survival (i.e., circulatory retention) rate decreases; however, it must be at least 75% during the approved life of the product [32,33].
3. Laboratory Investigation
a. As storage time increases, RBCs degrade in structural shape, aggregability, and metabolic function. The entire host of changes is referred to as the “storage lesion,” which contributes to limited post-transfusion RBC survival and potentially to impaired oxygen delivery via the microcirculation. The storage lesion may contribute to the morbidity associated with RBC transfusion [34,35].
b. A number of biochemical changes that occur over time in stored RBCs product have been well described. S-nitrosothiol (SNOs) levels change 2 to 3 hrs after collection; changes occurring after several days of storage progressive increases in lactate, potassium, and free hemoglobin and a decrease in pH [35]. Storage-induced reduction in RBC membrane structural integrity (reduced deformability and increased fragility) is thought to be related to intra-RBC energy source depletion. Release of cell-free hemoglobin and microparticles (MPs) can lead to increased consumption of nitric oxide (NO) [36]. Some investigators have proposed insufficient NO bioavailability as an explanation for the increased morbidity and mortality observed after transfusion [37,38].
c. Relevy and colleagues showed that routine cold storage damaged RBC membranes to increase stiffness, thereby potentially impairing flow properties. A continuous reduction in deformability was noted, ultimately producing rigid cells at the end of the storage period. The percentage of undeformable cells increased continuously starting as early as 2 wks after RBC collection. Increased RBC adherence was also noted. These findings suggest that transfusion of rheologically impaired RBCs might introduce morbidity risk, perhaps particularly to patients with cardiovascular disease [39].
d. In an animal model, Rigamonti and colleagues suggested that stored blood has a reduced capacity to deliver oxygen to brain tissue. They reported that fresh blood better restores cerebral oxygen delivery and tissue oxygen tension than stored blood does [40]. Recent studies suggest that stored RBCs may contribute to reported morbidity complications via release of RBC-derived MPs, which may facilitate thrombosis and lung injury [41–43].
5
4. Clinical Investigation
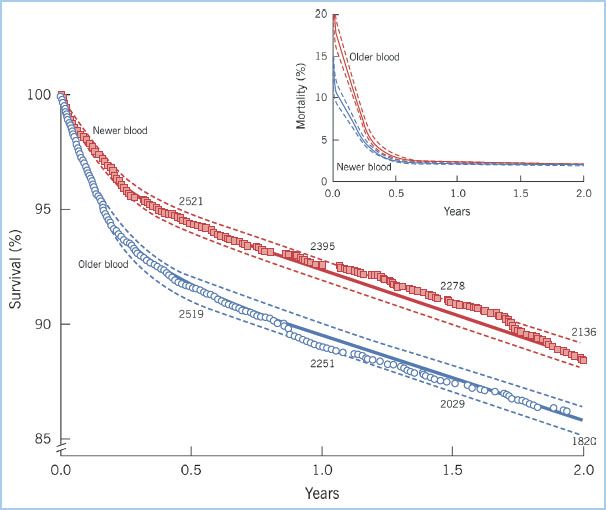
Clinical studies examining the role of RBC storage duration on patient outcomes report inconsistent findings, yet many have small sample size with heterogeneous patient populations. A large investigation in cardiac surgery reported an increased risk of postoperative complications and higher mortality in patients who were transfused RBCs with prolonged storage. In total 2,800 patients received RBCs with 14 days storage or less and 3,130 patients received RBC units with greater than 14 days storage. Risks for mortality, postoperative ventilatory support beyond 72 hrs, renal failure, sepsis, and composite adverse outcome were all higher in patients receiving older blood. Following risk adjustment, composite adverse outcome and lower survival were still found in patients who received “older” RBCs [30] (Fig. 18.5). A recent investigation in cardiac surgery reported a higher prevalence of new renal complications and length of hospital stay in patients receiving older RBCs [44].
Figure 18.5 Kaplan–Meier estimates of survival and death for patients given exclusively newer blood (stored for 14 days or less; squares) and patients given exclusively older blood (stored more than 14 days; circles). Survival was lower for patients who received older blood as compared to those receiving newer blood, especially during the initial follow-up period. Inset shows the same data presented as mortality at each time interval rather than cumulative survival. (Reproduced with permission from Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








