The logarithmic scale produces a 10-fold change in hydrogen ion concentration per unit change in pH. Modern blood gas machines rapidly measure several variables including pH, pCO2, PO2, oxyhaemoglobin concentration and ions (Na+, K+, Cl− and Ca2+). They calculate some variables including actual bicarbonate, standard bicarbonate and base excess.
The pH electrode measures pH. It consists of a silver measuring electrode which is coated with silver chloride and enclosed in a buffer solution of HCl which maintains the pH at 7. The electrode tip is composed of specialised H+ ion-sensitive glass. When placed in a solution the glass softens, forming a semi-solid gel membrane. Hydrogen ions diffuse into the outer layer of the glass, displacing ions such as lithium and sodium toward the inner layers. This creates a positively charged glass surface, which attracts anions (Cl−) from the buffer solution. An ion gradient is established within the glass electrode; this is dependent on the H+ concentration in the sample. Thus a potential difference is created. The potential difference is measured against a reference electrode that is connected by a lead wire and placed into the same sample being measured. The silver/silver chloride reference electrode is contained in a saturated 3 mol l−1 KCl solution, which gives it a fixed potential. It is encased in a non-permeable shell with a diaphragm at its tip, allowing continuity with the sample. Calomel electrodes were used previously, but are now avoided due to the toxicity of mercury.
Modifying the properties of the measuring membrane can make it specific to other ions including Na+, K+, Cl− and Ca2+. In this way, their concentrations can be measured using similar apparatus. Membranes used include the following.
Glass: framework of silicate with interstitial sites for H+.
Crystal: lattice containing defined gaps for ions of all types.
Polymers: contain an ionophore (molecule) that specifically binds the ion to be measured.
The pH electrode is shown below. 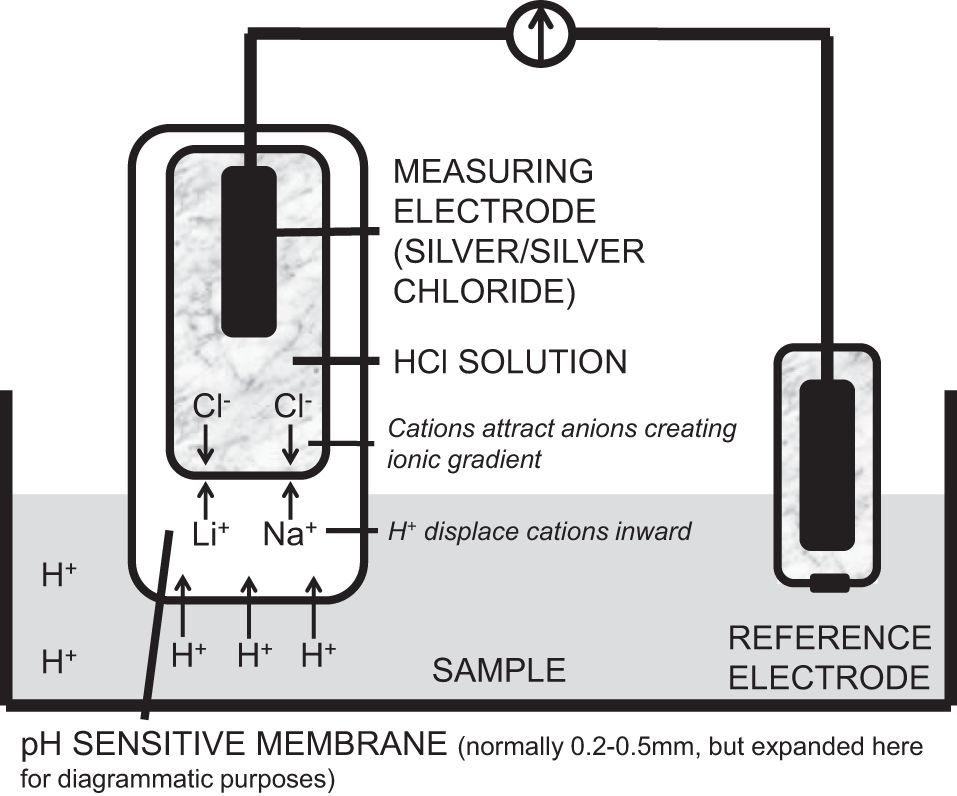
Electrodes are prone to certain errors.
Temperature affects the dissociation of H+ ions from bicarbonate. Therefore, the apparatus must be kept at 37°C.
Blockage of ion-sensitive channels or reference electrode diaphragm by precipitated silver chloride prevents functioning. Regular cleaning and maintenance are recommended.
Damage or leakage from the electrode into the sample negates potentials developed, requiring electrode replacement.
Ion selectivity of a membrane is not 100% specific. The presence of other ions in high concentrations can influence the results. Standard addition can be used to overcome this, whereby defined volumes of the ion to be measured are added to the sample in steps, and the effect measured. The initial concentration can then be calculated by extrapolation.
Drift affects the precision and accuracy over time, and can be prevented by regular 2-point calibration with buffer solutions of known pH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





