Fig. 10.1
(a) aSAH secondary to anterior communicating artery aneurysm rupture. Note the characteristic accumulation of blood in the basal cisterns. (b) CT angiography illustrates the anterior communicating artery aneurysm (arrow) in the patient in (a). Figure adapted with permission from Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396
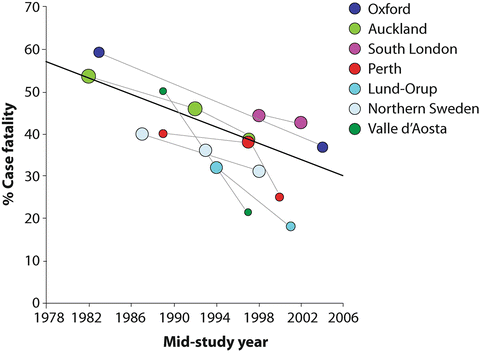
Fig. 10.2
Thirty-day case fatality rate over time. The 30-day case fatality rate after aSAH has decreased by 0.9 % per annum due to increased use of vascular imaging and reduced delays to treatment. Adapted with permission from Lovelock CE, Rinkel GJE, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage. Population-based study and systematic review. Neurology. 2010;74:1494–1501
Although mortality after aSAH has declined over the past few decades, the extent to which patients’ cognitive and functional outcomes have improved over the same time period remains unknown. A large proportion of aSAH patients—up to 76 % [10]—experience persistent cognitive deficits many years after aSAH. These cognitive deficits may lead to difficulties in aspects of functional independence such as return to work, managing finances, and housekeeping. Aneurysmal SAH-associated deficits in cognition and day-to-day functioning are particularly debilitating given that most patients are relatively young (mean age of 55 years [11]), are in their most productive years, and have major responsibilities with respect to work and family. This chapter describes the cognitive and functional deficits after aSAH and examines how clinical and neuroimaging factors influence cognitive and functional outcome.
Cognitive Outcome
Cognitive impairment is most frequent and severe within the first 3 months following aSAH [12], but residual cognitive dysfunction may persist for a lifetime [13]. The following section reviews the cognitive domains most commonly affected after aSAH: attention, memory, executive function, language, and visuospatial function.
Attention
Attention refers to the ability to allocate cognitive resources to a specific task, thereby producing focused behavior. Attention has many components, including selective attention (i.e., paying attention to a specific task), sustained attention (i.e., paying attention for a prolonged time period), and divided attention (i.e., paying attention to multiple tasks simultaneously). Several studies have found that among all cognitive domains, attentional deficits are the most frequent self-reported complaints by both aSAH patients and their relatives, with estimates as high as 70 % [14–20]. As a result of these attentional problems, patients often report difficulties with maintaining conversations, reading, watching television, and other tasks that involve cognitive processing [21].
The reported frequency and severity of attentional deficits after aSAH has been variable, however. A study by Ravnik et al. [22] found no correlation between subjective attentional impairment and performance on tests of attention; in fact, patients performed better on tests of attention than on tests of other cognitive domains. Other studies report the prevalence of attentional impairment, as measured by cognitive tests, to be modest, ranging from 3 to 19 % [23–28]. In one study, even patients with severe aSAH (i.e., grade 5 Hunt and Hess [29]) showed mild attentional deficits post-aSAH [30]. Hütter et al. [27] found that 70 % of aSAH survivors reported attention problems, but only 13 % were impaired on neuropsychological tests of attention. Discordance between self-reported cognitive deficits and results from objective cognitive tests may be attributed to patients misinterpreting their cognitive deficits as impairments of attention. Additionally, the high self-reported prevalence of attentional dysfunction may result from confounding factors such as depression [17] or excessive fatigue, the latter of which affects up to 75 % of aSAH survivors [14, 25, 31, 32].
Other studies have found that attentional deficits are common and widespread after aSAH, affecting upwards of 76 % of aSAH survivors [26, 33]. The extent of attentional impairment may depend on the timing of testing, with impairment more likely sooner after the hemorrhage, and on the specific type of attention being tested. Benke et al. [13] observed that while divided attention showed impairment after aSAH, sustained and short-term attention did not.
Several clinical variables affect the degree of attentional impairment post-aSAH. Greater aSAH severity, as measured by Hunt and Hess grade [29], the presence of blood in the Sylvian fissure, greater thickness of subarachnoid blood, as measured by Fisher grade [34], older age, intraventricular hemorrhage, hydrocephalus, and temporary clipping have been associated with poorer attentional function [16, 35–38]. Whether attentional function improves over time is controversial; some investigators claim no improvement over the 12-month period after aSAH [12, 33] while others show improvement over the same time period [39, 40].
Several investigators have correlated attentional function with neuroimaging. Mustonen et al. [41] found that cerebral perfusion heterogeneity, as measured by single photon emission tomography (SPET), at 1-week post-aSAH correlated with performance on attention tests at 1-year post-aSAH. Bendel et al. [42] found that hippocampal volumes after aSAH predicted performance on tests of attention 1-year post-aSAH.
Memory
In the aSAH outcome literature, memory is commonly stratified into different components, including visual memory, verbal memory, short-term memory, and long-term memory. Up to 57 % of survivors self-report memory problems after aSAH [14, 15, 19, 20, 43, 44]. Inability to remember new information and maintain short-term (working) memory are particularly common complaints [17, 44]. Verbal memory is commonly impaired in aSAH patients, the prevalence ranging from 14 to 68 % [12, 18, 20, 31, 45–49]. Likewise, deficits in visual memory are also frequent, with impairment prevalence ranging from 14 to 75 % [18, 20, 31, 33, 45–49].
Unlike attention, in which patients tend to overestimate the degree of subjective attentional dysfunction relative to neuropsychological tests, self-reported memory complaints often underestimate the degree of memory dysfunction as detected by neuropsychological impairment. Ljunggren et al. [18] found that 83 % of aSAH survivors had memory deficits on neuropsychological assessment, but only 58 % self-reported memory deficits on interview. Older age, fewer years of education, poorer neurological grade on admission (as measured by World Federations of Neurological Surgeons Grade [50]), hydrocephalus, ischemia, postoperative vasospasm, ruptured aneurysms in the anterior circulation, and thick subarachnoid blood in the anterior interhemispheric fissure and Sylvian fissures have been associated with poorer performance on tests of verbal and visual memory [33, 35, 51, 52]. Temporary clipping and partial resection of the gyrus rectus, meanwhile, have been associated with poorer short-term memory [27]. Some investigators have demonstrated a relationship between memory deficits and cerebral edema [35], but others have observed no correlation [53].
The wide range in the prevalence of memory impairment can be explained by the use of different standardized tests. Commonly used memory tests include the California Verbal Learning Test [54], the Visual Reproduction subtest of the Wechsler Memory Scale [55], and the Rey-Osterrieth Complex Figure—Recall subtest [56, 57]. Differences in difficulty and sensitivity to impairment between different memory tests may contribute to the variable rates of memory impairment. For instance, Mayer et al. [45] found that 31 % of aSAH survivors showed impaired visual memory on the Rey-Osterrieth Complex Figure—Recall subtest, whereas only 22 % showed impaired visual memory on the Visual Reproduction subtest. These findings suggest that the frequency of cognitive impairment after aSAH is determined in part by the particular test used to measure cognitive performance.
Time of testing in relation to the initial insult is also critical in determining aSAH-associated memory deficits. Powell et al. [12, 31] assessed verbal memory at 3, 9, and 18 months post-aSAH. Delayed verbal memory significantly improved from 3 to 9 months and from 9 to 18 months post-ictus, while immediate verbal memory showed no significant improvement in the same time period. Despite improvement in delayed verbal memory over 18 months, 14 % of aSAH patients still had significant delayed verbal memory impairments at 18-month follow-up. Although not all studies differentiate between immediate and delayed verbal memory, the finding that verbal memory improves over time has been replicated by others [39, 53, 58]. Visual memory may also improve over time [33, 39, 53], but results have been inconsistent [58]. Why verbal and visual memory improve over time remains unknown, although reduction of aSAH-associated intracranial pressure [59] and chronic inflammation may play a role [60]. Together, these results show that the prevalence of aSAH-associated memory impairment depends on the type of memory in question and on the length of the follow-up interval.
Although aSAH survivors often report a reduced capacity to learn new information [17], data by D’Esposito et al. [61] suggest that memory impairments are also characterized by an inability to recall information learned prior to aSAH. This form of retrograde amnesia, however, was only observed among patients with medial frontal lobe damage, mainly in arterial territories distal to the anterior communicating artery (ACoA). Given the role of the medial frontal lobe in initiation [62, 63], the inability to recall old information may be due to an inability to initiate memory retrieval. Indeed, Richardson [64] found that, on tests of object naming, aSAH patients had slower retrieval speeds compared to control participants.
The medial temporal lobes, a network of structures that includes the hippocampus together with the entorhinal, perirhinal, and parahippocampal cortices, are critically involved in memory function [65]. A correlation between left hemisphere infarctions and verbal memory impairment in patients with aSAH has been demonstrated [35, 66], but little is known about the specific brain regions in the left hemisphere responsible for verbal memory impairment in aSAH patients. Bendel et al. [42] performed magnetic resonance imaging (MRI) on aSAH survivors and found significantly reduced bilateral hippocampal volumes among aSAH patients relative to healthy controls at 1-year follow-up. Hippocampal volumes correlated with performance on one test of visual memory (the Visual Reproduction subtest of the Wechsler Memory Scale), but not with another visual memory test (the Rey-Osterrieth Complex Figure) or with tests of verbal memory. The investigators did not correlate memory impairment with changes in other brain regions, such as the frontal lobes. This may be important given the role of the frontal lobes in memory function [67] and given that frontal lobe injury is a common sequelae of aSAH [68]. Reduced frontal lobe integrity could account for the unexpected findings reported by Bendel et al. [42]. Indeed, Vilkki et al. [69] found that medial frontal lobe lesions on CT predicted poorer verbal memory in aSAH survivors.
Executive Function
Executive function is predominantly mediated by the frontal lobes [70] and encompasses higher-level cognitive abilities like planning, inhibition, problem-solving, and decision-making. Rather than fractionating executive function into its components, the majority of studies treat executive function as a unitary construct. Consequently, estimates of the frequency of executive dysfunction in aSAH survivors varies widely, ranging from 3 to 76 % [10, 12, 24, 31, 45–48, 71, 72]. Results from studies using neuropsychological assessments complement patients’ self-reported complaints; upwards of 30 % of aSAH survivors self-report a lack of initiative and a reduced capacity for planning and organizing [17, 18]. Executive dysfunction after aSAH is more pronounced in older patients, those with fewer years of education, and those with poorer neurological grade on admission [35, 36]. Similar to memory, a correlation between executive dysfunction and cerebral edema has been documented by some investigators [36], but not by others [53]. Surgical variables also affect outcome; Akyuz et al. [73] found that temporary vessel occlusion exceeding 9 min was a significant predictor of permanent long-term executive deficits.
Kreiter et al. [35] found that executive dysfunction was more severe among patients with ruptured anterior aneurysms than among those with posterior aneurysms. Findings from most other studies, however, suggest no relationship between ruptured aneurysm location and the profile of cognitive impairment [46, 53, 66, 74–76]. Interestingly, Manning et al. [77] found that aSAH patients who had ruptured ACoA aneurysms performed significantly better than patients with aneurysms at other locations on the Tower of London task [78], a test of executive function. Similarly, Papagno et al. [79] observed that patients with ACoA aneurysms performed better than those with aneurysms at other locations on tests of switching and inhibition. The relationship between aneurysm location and profile of cognitive impairment thus remains unclear.
In addition to sometimes causing focal lesions in proximity to the ruptured aneurysm, aSAH often leads to diffuse, global damage to brain tissue in poor-grade patients [10, 80–82]. Global injury is mediated, in part, by elevated intracranial pressure [80], reduction of cerebral blood flow and brain oxygenation, blood–brain barrier breakdown, and global cerebral edema (together these factors contribute to early brain injury; see [83]). Findings from an MRI study by Bendel et al. [84] support the “diffuse damage” hypothesis. The authors found that at 1-year follow-up, aSAH patients showed significantly reduced total grey matter and white matter volumes relative to control subjects. Furthermore, the extent of grey matter volume loss was associated with poorer cognitive test performance, including poorer performance on tests of executive function.
Not all neuroimaging studies, however, support the “diffuse damage” hypothesis. Several MRI studies suggest that executive dysfunction results from focal lesions rather than diffuse damage. Bendel et al. [85] found that 91 % of aSAH patients with executive dysfunction had frontal lobe lesions. Similarly, Martinaud et al. [47] showed that executive dysfunction in aSAH patients was exclusively correlated with lesions to the left frontal lobe, including the left superior frontal gyrus, the left middle frontal gyrus, and the left anterior centrum semiovale. Deficits in behavioral aspects of executive function (e.g., reduction of daily activities, irritability, hyperactivity), meanwhile, were correlated with lesions to the left ventral striatum. An exception to these findings is a study by Vilkki et al. [69] who showed that patients with infarction in the medial frontal lobe had impaired memory, but intact executive function. The relationship between executive dysfunction and frontal lobe injury may thus be qualified by the specific location of the lesion.
Although executive function and memory are often considered distinct cognitive domains, several studies indicate that these domains may interact. As mentioned earlier, D’Esposito et al. [61] observed that patients with medial frontal lobe injury showed deficits in retrieving previously learned information on a memory test. How can injury to the frontal lobes—a region implicated in executive function—affect performance on a memory test? Posterior-inferior midline frontal lesions may damage the septum, which has connection to the hippocampus and plays a role in working memory. Haug et al. [86] suggest that medial frontal lobe injury, which commonly occurs in patients with ACoA aneurysms [47, 85], may lead to deficits in initiation. Indeed, the investigators found that, compared to patients with aneurysms in other locations, these patients tended to perform poorer on the initial portions of memory tests. They also tended to commit more false-positive errors on memory tests, which may arise due to confabulation as opposed to genuine memory impairment. Deficits in executive function may thus “spill over” to affect performance on tests of other cognitive domains, thereby giving a distorted picture of a patient’s cognitive functioning.
The use of different neuropsychological tests, each of which probes a different executive function, may partially explain the high variability in executive dysfunction after aSAH. Proust et al. [48] reported that among aSAH survivors treated with endovascular coiling, 29 % showed impairment on the Wisconsin Card Sorting Task (a measure of cognitive flexibility [87]), while only 7 % showed impairment on the Stroop task (a measure of inhibition [88]). Different aspects of executive function may thus have different rates of impairment. In support of this hypothesis, deficits in cognitive flexibility, as measured by the Wisconsin Card Sorting Task, are found in 8–44 % of aSAH patients [24, 45–48, 71], whereas deficits in inhibition, as measured by the Stroop task, are found in 17–56 % of aSAH patients [46–48].
A study by Manning et al. [77] illustrates the importance of differentiating between different aspects of “frontal lobe” function. Manning et al. [77] evaluated executive function 26 weeks post-aSAH in patients who had made a “good recovery” according to the Glasgow Outcome Scale (GOS) [89]. Aneurysmal SAH survivors performed significantly worse than healthy controls on the Wisconsin Card Sorting, Tower of London, and Stroop tasks, while performance on the Cognitive Estimates [90] task was within normal limits. Each of the tests used by Manning et al. [77] captures a slightly different executive “frontal lobe” function: the Tower of London task assesses planning and problem-solving, the Stroop task assesses ability to inhibit impulsive responding, while the Cognitive Estimates task measures ability to make judgments and estimations. These results indicate that some executive functions are impaired in aSAH survivors (e.g., cognitive flexibility, planning, problem-solving, inhibition), while others are intact (e.g., judgment, estimation). Furthermore, the data suggest that patients classified as having made a good recovery on the GOS may still experience profound cognitive deficits more than 6 months after aSAH. This is consistent with earlier studies showing that up to one-third of aSAH survivors who have made a good recovery on the GOS experience persistent neuropsychological impairment [91].
Different aspects of executive function may have different rates of recovery. Haug et al. [53] found that while inhibition improved within 1 year after aSAH, cognitive flexibility and attention did not. Results from longitudinal studies, however, must be interpreted with caution, as tests of executive function may be subject to practice effects (e.g., Stroop test [92], Trail Making Test [92], Wisconsin Card Sorting Test [93]). Studies that found improvement in response inhibition and cognitive flexibility over time [39, 58] used the Stroop test and Trail Making Test, both of which may have practice effects [92]. These findings further illustrate the importance of distinguishing between different executive functions.
Language
Language function involves comprehension and expression of meaningful written and oral information. The frequency of language impairment among aSAH survivors is highly variable, ranging from 0 to 76 % [10, 12, 24, 31, 45–48, 71]. Few studies have examined patients’ self-reported language complaints; Passier et al. [17] observed that only 8 % of aSAH survivors self-reported deficits in speaking or writing. Interestingly, language function appears to be critical for predicting patients’ perceptions of recovery. Chahal et al. [94] found that 52 % of patients felt they had made a complete recovery from the effects of aSAH 5 years post-ictus. Patients who reported making a full recovery, however, tended to perform better on tests of language and verbal functioning than patients who reported not making a full recovery, suggesting that linguistic function is key for patients’ perception of recovery. Older age, fewer years of education, aneurysms in the anterior circulation, and left hemisphere infarction are significant predictors of poorer language function after aSAH [35, 69].
Language function improves significantly within the first 3 months after aSAH [58] and continues to improve in the 18 months after hemorrhage [12, 31, 58]. Longitudinal studies have typically used the verbal and semantic fluency task, which is not subject to practice effects [92, 93]. Mavaddat et al. [10] followed aSAH patients with a favorable neurological outcome (i.e., GOS categories of moderate disability and good recovery) between 6 and 24 months after aSAH. Despite patients having a favorable neurological outcome, 76 % of aSAH survivors were significantly impaired on the verbal and semantic fluency task, indicating that overall patient status, as measured by the GOS, may not be an accurate indicator of language dysfunction after aSAH.
Visuospatial Function
Visuospatial function refers to one’s capacity for perception and spatial reasoning. According to several investigators [35, 45], visuospatial function is the least frequently impaired domain after aSAH, with an impairment prevalence of approximately 25 %. Furthermore, any visuospatial impairments that patients do exhibit tend to be mild and close to normative limits [40, 53]. Anterior aneurysms, the presence of blood in the Sylvian fissure, edema, older age, and right hemisphere lesions are significant predictors of poorer visuospatial function after aSAH [35, 69, 95]. Unlike other cognitive functions, deficits in visuospatial function persist over the 1-year period after aSAH [40, 58]. Although less is known about the integrity of visuospatial function after aSAH compared to other cognitive domains, several studies indicate that one test of visuospatial function—the Rey-Osterrieth Complex Figure Test—is the most sensitive test of long-term cognitive functioning in aSAH survivors (i.e., up to 5 years post-ictus) [40, 43].
Functional Outcome
Neuropsychological assessments attempt to characterize performance in individual cognitive domains. Day-to-day behavior, however, involves an interplay between many different cognitive domains rather than single cognitive domains working in isolation. Functional outcome encompasses activities of daily living (ADLs), instrumental activities of daily living (IADLs), and the ability to return to work. Each of these domains will be addressed in turn.
Activities of Daily Living
ADLs are those one performs for self-care [96]. Examples of ADLs include feeding, grooming, dressing, bathing, personal hygiene, and toileting. Aneurysmal SAH-associated ADL deficits are less common compared to cognitive deficits, with impairment ranging from 4 to 12 % [25, 72, 97, 98]. Severity of aSAH, as measured by Hunt and Hess grade [29], and hydrocephalus have been shown to predict ADL impairment after aSAH [99]. Performance on ADLs is key for patients’ perceptions of recovery; one recent study showed that ADL performance best differentiated aSAH survivors who reported making a complete recovery from those who reported disability [94]. Common ADL impairments at hospital discharge include incontinence and maintaining personal hygiene [100], but whether these deficits extend to longer follow-up periods remains unclear. Berry et al. [51] showed that the degree of ADL dysfunction correlated with the extent of memory impairment. Similarly, Mayer et al. [45] found deficits of visual memory, visuospatial function, and psychomotor functioning to be significantly associated with impairment in ADL at 3 months post-aSAH, thereby highlighting the link between cognition and functional outcome.
One concern with measuring ADLs after aSAH is that of ceiling effects. In studies that measure ADLs using the Barthel Index [101], over 95 % of assessed patients achieve the highest possible score [102]. Furthermore, many ADL measures (e.g., the Barthel Index, the Katz Index of Independence in ADLs [103]) rely heavily on patient self-report, the accuracy of which is uncertain. For instance, patients may be uncomfortable or embarrassed at disclosing difficulties with toileting or bathing. Thus, the specific profile of ADL impairments after aSAH remains unclear.
Instrumental Activities of Daily Living
IADLs are more complex than ADLs and include tasks like managing finances, shopping, and housekeeping [104]. IADLs are more frequently impaired than ADLs after aSAH, with an estimated prevalence of 44–93 % [12, 31, 105, 106]. Of all IADLs, driving appears to be the one in which patients show the most impairment, with up to 60 % of aSAH survivors requiring assistance [107]. Housekeeping difficulties are also common, as only 51 % of aSAH survivors are capable of managing their households without any limitations [15]. Deficits of visual memory, visuospatial function, language function, and psychomotor function are associated with IADL impairment [45, 94]. Similar to ADLs, the instruments used to measure IADLs may not be sensitive to the cognitive difficulties aSAH survivors may experience. While driving on a busy city street, for instance, aSAH survivors might experience transient lapses of concentration or feelings of being overwhelmed. Despite these subtle difficulties, however, patients would receive the maximum favorable score on the Mode of Transportation subscale of the Lawton IADL scale [104], as their performance fulfills the criteria of traveling independently. Furthermore, commonly used measures of IADL performance, like the Lawton IADL scale, often employ a binary scoring system that does not allow for different gradients of IADL performance. IADL measures also rely heavily on self-report, which may not accurately reflect patients’ true performance capabilities. Patients may be reluctant to disclose difficulties with driving, for instance, because they fear having their driver’s license suspended. Additional research is needed to characterize the specific IADL profile in aSAH survivors.
Return to Work
One of the most important components of day-to-day functioning is the ability to return to one’s previous occupation. This is especially important in the case of aSAH, as many aSAH survivors are young and have financial responsibilities and families to support. Among patients who were employed prior to aSAH, up to 40 % are unable to return to their previous occupation [14, 66, 80, 97, 108–111]. Other studies have found less favorable results, demonstrating that only 6–17 % of patients return to their previous occupation [32, 106, 112]. Even among those who do return to work, however, up to 59 % report reduced working capacity [15]. Return to work is an important issue even among patients with mild cognitive deficits; Haug et al. [30] found that only 21 % of previously employed aSAH patients with mild cognitive dysfunction were able to resume full-time employment. Patients frequently return to jobs with less responsibility, and they must often work fewer hours [14, 19, 32, 110, 111]. The impact of fatigue is profound, for up to 34 % of patients require daytime naps in order to function normally [20]. Research by Cedzich and Roth [15] indicates that, among patients who return to work, most do so between 6 and 12 months post-aSAH. Interestingly, patients’ employment status prior to aSAH has been shown to predict the extent of cognitive impairment 1-year post-ictus. Haug et al. [30] observed that the fraction of patients working full-time prior to hemorrhage was less among patients with severe cognitive impairment than among those with mild cognitive impairment 1 year after aSAH. Although only correlational, these results suggest that one’s resources before aSAH influence the course of post-stroke cognitive recovery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







