Category
BMI (kg/m2)
Underweight
<18.5
Normal weight
18.5–24.9
Overweight
25–29.9
Obesity class 1
30–34.9
Obesity class 2
35–39.9
Extreme (severe) obesity class 3
≥40
Obesity is a multifactorial disease with important societal and public health implications. Although the fundamental mechanism is an imbalance between caloric intake and energy expenditure, there is physiologic, biochemical and genetic evidence that obesity and severe obesity are not simple disorders of willpower. In addition, several factors have contributed to the observed increasing rates of obesity over the last 40 years: major changes in the availability, composition, preservation methods and cost of available food products in the United States and worldwide, urbanization, and other changes in modern life style. Important examples are the increase in consumption of sugar, high-fructose corn syrup, fats, and consumption of grain-fed over grass-fed livestock meats during recent decades [8, 9]. Other factors that add to the complexity of the matter include the United States government-policy on farm subsidies, the marketplace involving food manufacturers, revenues from beverage and processed foods, among many others. The low cost of government-subsidized commodities like corn and soybeans, make sugars and fats some of the cheapest food substances to produce. At the same time, prices for fruits and vegetables, grown with relatively little government support, have risen nearly 40 % in the past 20 years [10].
Obesity is associated with severe metabolic disorders, poor quality of life and decrease in life span [11]. While the association between obesity and type II diabetes, hypertension, hypercholesterolemia, and obstructive sleep apnea are well established, many other medical, psychiatric, and oncologic disorders are closely associated with obesity (Table 15.2). The comorbidities associated with obesity can have a massive impact on the overall health and psychological well-being of patients. Most of these comorbidities will correct to some degree with weight loss [12, 13].
Table 15.2
Obesity associated co-morbidities
Cardiovascular | Hypercholesterolemia Hypertension Cardiomyopathy |
Pulmonary | Obstructive sleep apnea Obesity hypoventilation syndrome Pickwickian syndrome Asthma |
Gastrointestinal | Non-alcoholic steatohepatitis Cholelithiasis Gastroesophageal reflux disease |
Endocrine | Type II diabetes mellitus |
Orthopedic | Knee arthropathy Low back pain Weight bearing joint pain |
Psychologic | Depression Anxiety |
Genitourinary | Stress incontinence Polycystic ovarian syndrome Infertility Gestational diabetes Poor maternal and fetal outcomes |
Oncologic | Endometrial cancer Breast cancer Prostate cancer Colorectal cancer Esophageal cancer Hepatocellular carcinoma Ovarian cancer |
While societal changes and treatment strategies aimed at prevention of obesity are essential to stem the rising rates of obesity, treatment for the millions of individuals with established obesity and severe obesity are essential. For individuals with severe obesity, non-surgical treatment modalities have high rates of failure and bariatric surgery should be offered as a therapeutic option. Bariatric surgery is currently the most effective treatment for severe obesity and has been shown to be cost-effective at approximately $18,000.00 per Quality Adjusted Life Year (QALY) [14]. When the operation is carried out in high-volume centers by expert surgeons, it is associated with a low risk of peri-operative and long-term complications [15], major and sustained weight loss in most patients [16], improvements in or cure of obesity associated diseases including type 2 diabetes [17], a better quality of life [18] and increased life expectancy resulting from fewer cardiovascular and cancer- related deaths [19, 20].
Historically, there were a number of surgical procedures performed for the treatment of obesity. Many of these procedures, like jejunoileal bypass, were associated with high rates of long-term complications and treatment failure and are thus no longer performed. Others, such as silicone ring gastroplasty, horizontal gastric bypass and vertical banded gastroplasty are seldom performed nowadays. They have fallen out of favor due to high rates of weight recidivism and/or the development of more standardized procedures that can be done using minimally invasive techniques. In the early 1990s roughly only 20,000 bariatric procedures were performed annually in the United States [21]. This number increased to approximately 180,000 by 2006 and continues to climb slowly [21]. A worldwide survey [1] shows that the total global number of bariatric procedures increased from 146,301 in 2003 to 340,768 in 2011 (Fig. 15.1). Many factors have led to this dramatic increase in the utilization of bariatric surgery in the United States, and include the introduction of laparoscopic minimally invasive techniques, the increasing rates of obesity, the recognition of obesity as a health hazard, the poor outcomes with behavioral and medical management of obesity and the reproducible good outcomes with bariatric surgery in high volume centers [22]. However, weight loss results vary significantly with the different surgical techniques in practice, and the mechanisms promoting weight loss after bariatric surgery are still under scrutiny.
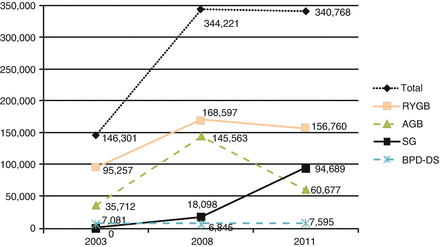

Figure 15.1
Trends in numbers of procedures worldwide from 2003 to 2008 to 2011 (Adapted from Buchwald and Oien [1]). RYGB Roux-en-Y gastric bypass, AGB adjustable gastric band, SG sleeve gastrectomy, BPD-DS biliopancreatic bypass-duodenal switch
Nowadays, two of the most common performed procedures for the treatment of obesity worldwide are Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). A review of the techniques and outcomes of these procedures will be described in this chapter.
Indications for Bariatric Surgery
A decision favoring operative treatment must consider the potential benefits and risks of general anesthesia and an abdominal operation. Assessment of a patient’s readiness for operation is a clinical judgment that should be made by a surgeon experienced in the operative management of obese patients in conjunction with other health allied professionals with expertise in the evaluation and management of obesity, such as a mental health care professional and a dietician.
In 1991, the National Institutes of Health (NIH) convened a panel of experts from a variety of specialties to review what were then the current data on the treatment of obesity in order to define a patient population that would most benefit from bariatric surgery. The NIH consensus criteria stated that in order to qualify for surgery, patients must have a BMI >40 kg/m2 or a BMI of 35–39.9 kg/m2 with specific obesity-associated comorbid conditions such as severe sleep apnea, type 2 diabetes mellitus (T2DM), or hypertension [23]. They also recommended that patients must be well-informed, self-motivated and of acceptable operative risk. Additionally, they must have failed previous non-surgical treatment options, which include integrated diet, exercise, behavioral modification and psychological support [23]. These criteria were adopted by the Centers for Medicare and Medicaid Services and eventually by most private insurance companies, and they remain to date the accepted indications for operative treatment.
Age alone is no longer a contraindication to operative treatment if a patient has comorbidities that would benefit from sustained weight reduction. Bariatric surgery in adolescents is still considered experimental, and should be done only in specialized medical centers under the supervision of an Institutional Review Board.
While outcomes following bariatric surgery are superior to all other forms of obesity treatment, bariatric procedures may fail over the long term if there is no significant patient motivation to change lifestyle. In addition, it is recommended that bariatric surgical programs should include a multi-disciplinary team for patient evaluation and a structured pathway for pre- and post-operative counseling and medical evaluation. Team members should include bariatric surgeons, health pyschologists, nutritionists, and exercise physiologists. Patients should be advised and agree (1) to comply with lifestyle changes; (2) adhere to nutritional supplement prescriptions; (3) comply with eating behavior modifications; and (4) participate in long-term monitoring and follow-up evaluation.
Laparoscopic Roux-en-Y Gastric Bypass (RYGB)
RYGB was initially described by Drs. Mason and Ito at the University of Iowa in 1969 [24]. Many individuals contributed to the introduction and standardization of laparoscopic minimally invasive techniques. Noteworthy was the work of Dr. Allan Wittgrove with the publication of the first series of laparoscopic RYGB for morbid obesity in 1994 [25]. Since then, most bariatric procedures have been performed utilizing minimally invasive techniques with similar weight loss and resolution of obesity associated disease outcomes [18], but significant decreases in morbidity compared to open bariatric procedures [15].
RYGB combines restriction caused by a small gastric pouch with several other physiologic changes caused by the Roux-en-Y anatomy, because RYGB prevents contact of the food bolus with most of the stomach and the duodenum while allowing for early delivery to the proximal jejunum. Caloric restriction with RYGB leads to negative energy balance and different degrees of weight loss and decreases in total fat and lean body mass and fat in the liver, visceral, and peripheral tissues [12, 26]. These changes are also associated with a decrease in hepatic glucose production and an increase in insulin sensitivity in the liver, muscle, and adipose tissue [27]. RYGB is also known to promote postprandial changes in many gastrointestinal (e.g., ghrelin, glucagon-like-peptide 1, glucose-dependent insulinotropic polypeptide, peptide YY) and pancreatic (insulin, glucagon, pancreatic polypeptide) hormone levels [27–29]. These hormones affect gastric emptying, glucose regulatory mechanisms, central nervous system hunger, and satiety mechanisms [30] in addition to changes in diet induced thermogenesis [31], bile acid metabolism [32] and gut microbiota composition [33]. It has been recently suggested that persons undergoing RYGB may also have increases in weight-adjusted resting energy expenditure after substantial weight loss has occurred [34, 35], a factor that might support the sustained weight loss after RYGB.
Operative Technique of Laparoscopic RYGB
Positioning and Anesthesia
Patients undergoing laparoscopic RYGB are at high risk for deep venous thromboembolism (DVT) and pulmonary embolism. DVT prophylaxis with subcutaneous heparin (unfractionated or low molecular weight heparin) 30 min prior to the induction of anesthesia should be the standard in all cases. Lower extremity sequential compression devices should also be on and operational prior to induction. Proper patient positioning on an appropriate table is critical to avoid injuries. Bariatric patients may be at higher risk for brachial plexus injuries, especially with prolonged cases. Arms should be padded and carefully secured. Arms should be flexed anteriorly when out at the sides on wedges to prevent brachial plexus stretch. Steep reverse Trendelenburg during certain phases of laparoscopic RYGB can be extremely helpful for exposure and the use of a foot board minimizes the chance of a patient sliding off the table. An anesthesia team that is experienced and comfortable intubating extremely obese patients with difficult airways using a variety of techniques is critical. We routinely administer a dexmedetomidine infusion (Precedex, Hospira, Lake Forest, IL) beginning 30 min before the anticipated completion of the procedure and in the recovery room. Dexmedetomidine is an alpha-2 receptor agonist with sedative and analgesic sparing properties. We have found that patients who receive this infusion require less narcotic pain medication for pain control after surgery and are discharged to home sooner following laparoscopic gastric bypass [36].
Description of Procedure
The technique for laparoscopic RYGB varies from center to center. The most common variations relate to the position of the alimentary limb (Roux-limb) and the technique used to create the gastrojejunostomy. The alimentary limb can be placed anterior to both the colon and the gastric remnant (antecolic), posterior to the colon and anterior to the gastric remnant (retrocolic-antegastric), or posterior to both (retrocolic-retrogastric). Most centers in the US now prefer the antecolic approach; however a retrocolic route maybe needed in selected patients to allow for the gastrojejunostomy to be done without tension. Using the retrocolic approach routinely is an option, but that approach creates an additional space for an internal hernia and has been associated with increased risk of internal hernias [37]. The gastrojejunostomy is most often constructed using surgical staplers (either circular staplers or linear staplers) or using hand-sewn techniques with sutures. There are advantages and disadvantages to each technique, and experienced surgical teams can achieve good and safe outcomes in a timely manner with all of these methods. Our standard technique involves the antecolic alimentary limb and a circular stapler gastrojejunostomy. For the circular stapler gastrojejunostomy the stapler anvil maybe 21 or 25 mm and passed either using a transgastric or trans-oral technique.
1.
Port placement
We employ a six port technique for laparoscopic RYGB. Initial abdominal access is usually obtained using a direct trocar insertion technique without pneumoperitoneum using 10-mm optical viewing trocar and a 0° scope in the left paramedian supra-umbilical location [38]. A 12 mm port is placed in the right flank. Additional 5 mm ports are then placed in the subxiphoid area, left upper quadrant and left flank. A third 12 mm port is then placed in the left lower quadrant and that incision will also be used to insert the circular stapler (Fig. 15.2). The first assistant stands on the patient’s left side and holds the camera with his/her left hand and assists through the left lateral port with his/her right hand. The surgeon stands on the patient’s right side.
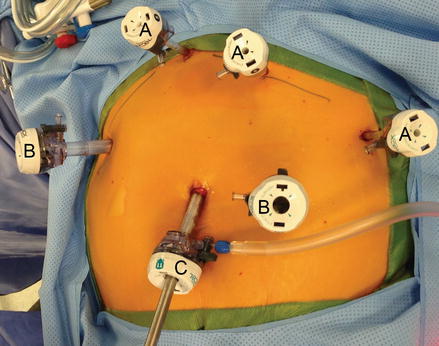

Figure 15.2
Ports placement for laparoscopic gastric bypass (A 5 mm trocar, B 12 mm trocar, C 11 mm trocar)
2.
Creation of the Alimentary limb and Jejuno-jejunostomy
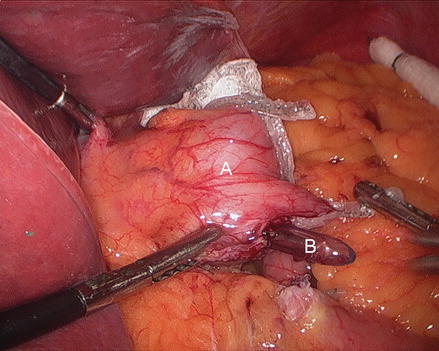
We routinely create the alimentary limb and jejuno-jejunostomy as the initial step. The omentum is reflected cephalad and tucked in the upper abdomen and the transverse colon elevated. After elevating the transverse mesocolon, the ligament of Treitz is identified at the base of the transverse mesocolon (Fig. 15.3). The ligament of Treitz is clearly identified and the bowel is run for about 50 cm to find a jejunal loop that can reach the upper abdomen without tension. The jejunum is then transected using an endoscopic linear cutting stapler using 2.5 mm height staplers (Fig. 15.4). The mesentery between the bilio-pancreatic and alimentary limb is then divided for about 2 cm with an ultrasonic dissector. In rare cases, a more complete division of the mesentery is needed. The alimentary limb is marked with two clips to facilitate later the identification when creating the jejuno-jejunostomy and prevent a Roux-en-“O” error (mistakenly connecting the alimentary limb back to itself). The best length of the alimentary limb is still a matter of debate; we choose the alimentary limb length based on the patient’s BMI: 100 cm for a BMI < 50 kg/m2 and 150 cm for a BMI ≥ 50 kg/m2 [39]. Importantly, the alimentary limb should be moved and placed to the left of the bilio-pancreatic limb and angle of Treitz (Fig. 15.5). The jejuno-jejunostomy is then created by connecting the biliopancreatic limb to the 100 or 150 cm location of the alimentary limb. Enterotomies are created in each segment with the ultrasonic shears (Fig. 15.6). The enterotomy in the bilio-pancraetic limb is done at the top of the cut staple line and the enterotomy in the alimentary limb should be done in the left lateral side of the small bowel mid-way between the mesentery and the anti-mesenteric border to prevent narrowing the lumen when closing the common enterotomy (Fig. 15.6). An endoscopic linear cutting stapler of 60 mm length with 2.5 mm height staples is inserted down each lumen and fired, creating a side-to-side functional end-to-end jejunojejunostomy (Figs. 15.7 and 15.8). The staple lines are inspected for hemostasis. The opening in the bowel is closed with an additional load of the endoscopic linear cutting stapler with 3.5 mm height staples, taking care not to compromise the caliber of the lumen of the jejunojejunostomy (Fig. 15.9). An anti-kinking stitch is placed to prevent obstruction at the jejunojejunostomy (Fig. 15.10). The mesenteric defect is then closed with a running locking 2–0 braided polyester suture from the anastomosis to the base of the mesentery (Fig. 15.11).
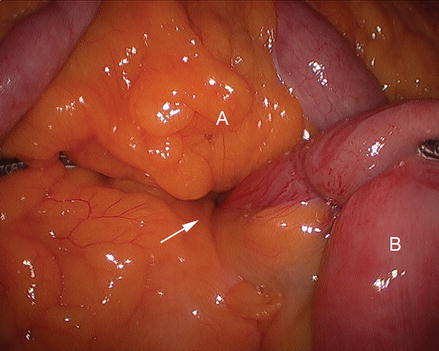
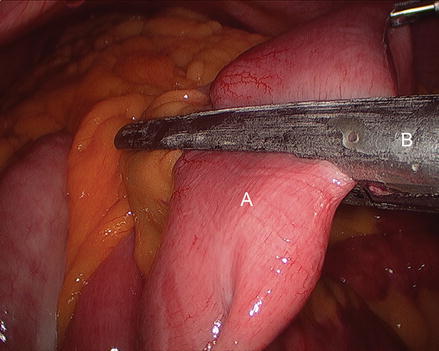
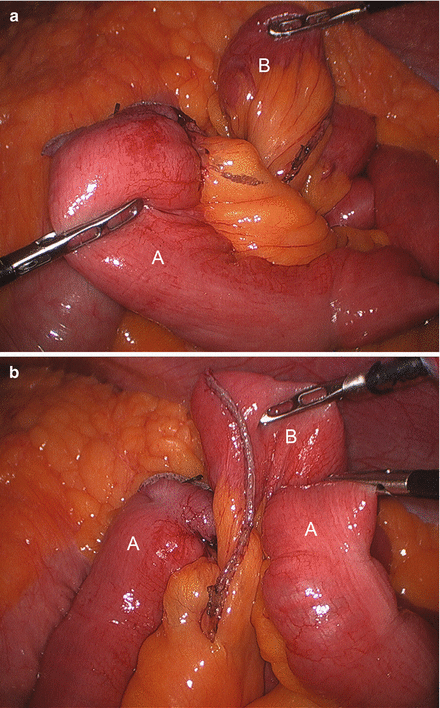
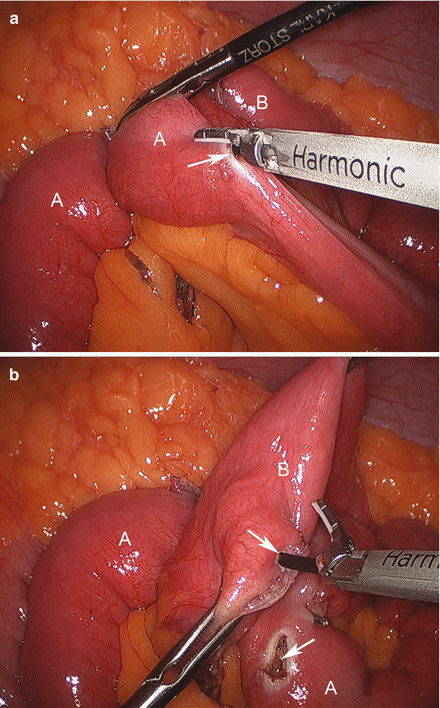
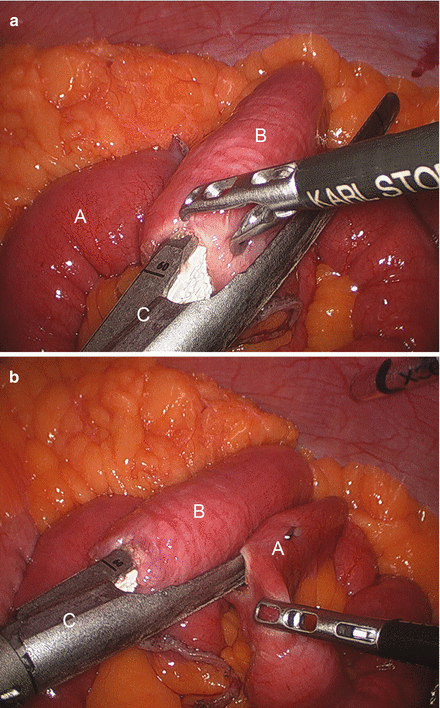
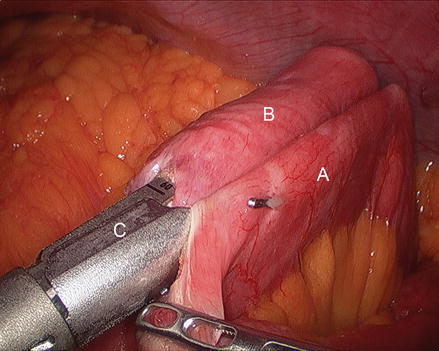
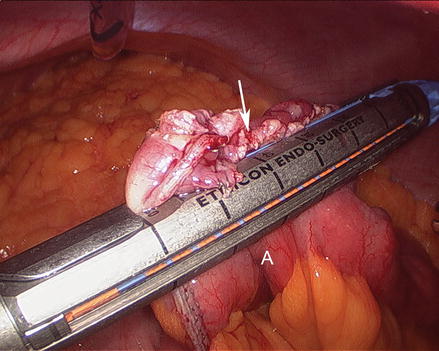
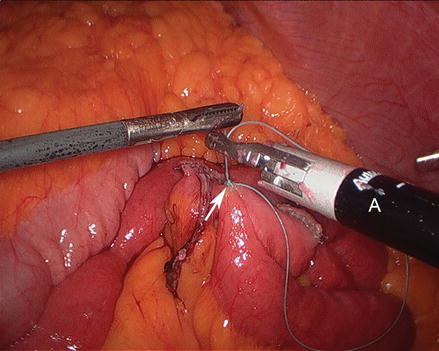
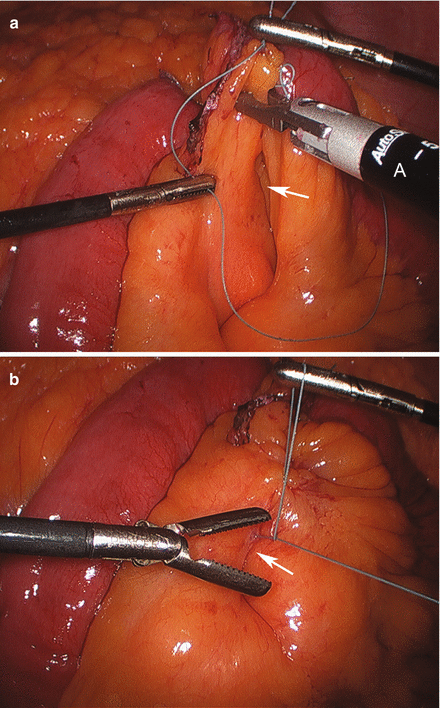

Figure 15.3
Identifying the ligament of Treitz (Arrow), (A transverse mesocolon, B jejunum)

Figure 15.4
Transecting the jejunum using an endoscopic linear cutting stapler (A jejunum, B endo GIA stapler)

Figure 15.5
Placing the alimentary limb (A) to the left of the bilio-pancreatic limb (B)

Figure 15.6
Creating enterotomies (Arrows) in the alimentary limb (A) and bilio-pancreatic limb (B)

Figure 15.7
Placing the bilio-pancreatic limb (B) and the alimentary limb (A) in the linear stapler (C)

Figure 15.8
Creating a side-to-side jejunojejunostomy using an endoscopic linear cutting stapler (A alimentary limb, B bilio-pancreatic limb, C endo GIA Stapler)

Figure 15.9
Closing the opening of the entero-enterostomy (Arrow) using an endoscopic linear cutting stapler

Figure 15.10
Placing an anti-kinking stich (Arrow) at the jejunojejunostomy

Figure 15.11
Closing the mesenteric defect (Arrow) using suturing device (A)
Closing Peterson’s space (between the mesentery of the alimentary limb and the transverse colon when the antecolic technique is used) is advocated by many. We split the omentum up the middle from the mid transverse colon using ultrasonic shears (Fig. 15.12). The alimentary limb is brought anterior to the mid-transverse colon and into the proximal abdomen between the leaves of the omentum. At this point, attention is turned to creating the gastric pouch.
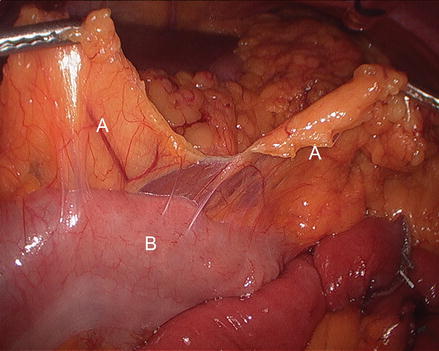

Figure 15.12
Dividing the omentum (A) in the middle of the transverse colon (B)
3.
Creation of the Gastric Pouch
The patient is positioned in steep reverse Trendelenburg at this time to facilitate exposure of the proximal stomach. A liver retractor is inserted through a subxiphoid 5 mm incision and secured with a mechanical arm retractor to lift the left lobe of the liver anteriorly. We start by dissecting the gastroesophageal junction fat pad off the left crus of the diaphragm at the angle of His. This makes later visualization of the proximal stomach during gastric pouch creation a bit easier. We then dissect between the proximal lesser curve gastric wall and the lesser curve neurovascular bundle and into the lesser sac (Figs. 15.13 and 15.14). This location is typically between the fat pad and the visible first lesser curve vessel on the anterior proximal stomach (about 5 cm distal from the estimated location of the gastroesophageal junction). A 3.5 mm height, 60 mm linear stapler is initially fired to divide the stomach in a right-to-left horizontal plane (Fig. 15.15). Next, two or three 3.5 mm height, 60 mm linear staplers are used to create a 30 ml gastric pouch divided at the level of the angle of His (Fig. 15.16). Specific care is always taken not to leave redundant gastric fundus on the side of the gastric pouch.
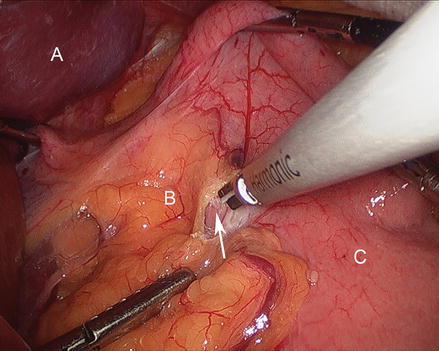
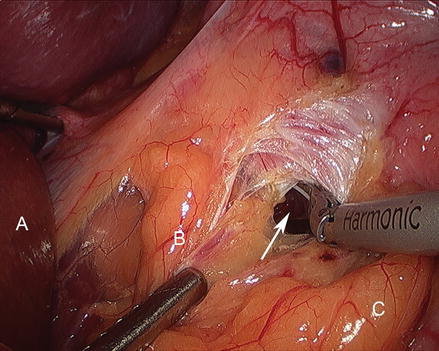
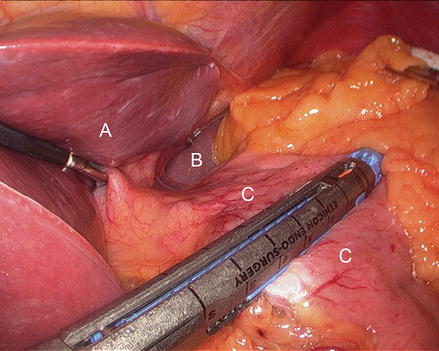
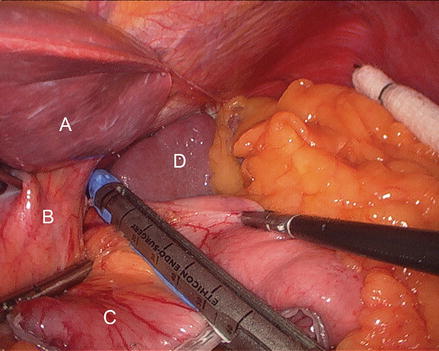

Figure 15.13
Dissecting into the lesser sac (Arrow) (A liver, B gastrohepatic ligament, C stomach)

Figure 15.14
Dissecting into the lesser sac (Arrow) (A liver, B gastrohepatic ligament, C Stomach)

Figure 15.15
Creating the gastric pouch with a linear cutting stapler (A liver, B spleen, C stomach)

Figure 15.16
Creating a gastric pouch by dividing the proximal stomach at the level of angle of His (A liver, B left crus of diaphragm, C gastric pouch, D spleen)
4.
Creation of the Gastrojejunostomy
We prefer the trans-oral placement of the anvil and with the anvil delivered through the cut staple line of the gastric pouch. We routinely use a 21 mm anvil connected to a 3.5 mm height stapler. However, a 21 mm circular stapler (as opposed to a 25 mm circular stapler) should only be used when the trans-oral technique is used [40]. The transgastric 21 mm stapler technique has been associated with higher stricture rate in ours and others experience [41]. The gastrojejunal anastomosis technique includes passing the 21 mm anvil (OrVilTM, Autosuture, Norwalk, CT, USA) trans-orally and through a small opening in the stapled gastric pouch (Fig. 15.17). The OrVilTM 21 mm device is a pre-packaged commercially available device (OrVilTM, Autosuture, Norwalk, CT, USA). It combines the anvil head, secured in the tilted position, mounted on a 90-cm long polyvinyl chloride (PVC) delivery tube and secured to the tube with a suture. The PVC delivery tube is inserted through the patient’s mouth, delivered through a small opening in the stapled gastric pouch and pulled from left lower quadrant port sites to assist bringing the anvil shaft into the gastric pouch staple line. A critical step of the procedure is then passing the tilted anvil head attached to the delivery tube through the posterior pharynx into the esophagus. We recommend that the anesthesiologist and an assistant are present for this portion of the procedure. The anvil head should be generously lubricated, and its convex side directed and maintained towards the hard palate. Once the anvil enters the posterior pharynx, elevating the mandible, similar to a Jaw thrust maneuver, and briefly deflating the balloon of the double-lumen endotracheal tube, facilitates the anvil passage into the esophagus. Once the anvil shaft has been exteriorized through the gastric staple line, the suture that holds it to the delivery tube is cut and the tube is disconnected from the anvil while holding the anvil in place (Fig. 15.18). The anastomosis is completed by joining the anvil to the end-to-end circular stapler (EEA XL 25 mm with 3.5 mm staples, Autosuture, Norwalk, CT, USA) inserted into the small bowel (Figs. 15.19, 15.20 and 15.21). Then, the EEA stapler and anvil were removed, the anastomosis inspected and the small bowel opening closed using an additional firing of a 2.5 mm linear stapler (United States Surgical Corporation, Norwalk, CT, USA) (Fig. 15.22).