KEY POINTS
Adults with bacterial meningitis usually present clinically with fever, headache, meningismus, and/or signs of cerebral dysfunction; elderly patients, however, may present with insidious disease manifested only by lethargy or obtundation, variable signs of meningeal irritation, and no fever.
Occasionally, a patient with acute bacterial meningitis has a low cerebrospinal fluid (CSF) white cell count despite high bacterial concentrations in CSF; therefore, a Gram stain and culture should be performed on every CSF specimen, even if the cell count is normal.
Neuroimaging techniques have little role in the diagnosis of acute bacterial meningitis. However, computed tomography (CT) should be performed before lumbar puncture when a space-occupying lesion of the central nervous system (CNS) is suspected. Clinical features for which patients should undergo CT scanning prior to lumbar puncture are immunocompromise, a history of CNS disease, a history of seizure within 1 week before presentation, papilledema, and specific neurologic abnormalities.
Empirical antimicrobial therapy, based on the patient’s age and underlying disease status, should be initiated as soon as possible in patients with presumed bacterial meningitis; therapy should never be delayed while diagnostic tests such as CT are awaited.
Adjunctive dexamethasone therapy has been shown to decrease the morbidity rate in infants and children with acute Haemophilus influenzae type b meningitis and, if commenced with or before antimicrobial therapy, may also be beneficial for pneumococcal meningitis in childhood. Adjunctive dexamethasone is also associated with decreased morbidity and mortality rates in adults with pneumococcal meningitis when administered before the first dose of antimicrobial therapy.
Fewer than 50% of patients with brain abscess present with the classic triad of fever, headache, and focal neurologic deficit; the clinical presentation of brain abscess in immunosuppressed patients may be masked by the diminished inflammatory response.
The diagnosis of brain abscess has been revolutionized by the development of CT; magnetic resonance imaging offers advantages over CT in the early detection of cerebritis, cerebral edema, and satellite lesions.
Aspiration of brain abscess under stereotaxic CT guidance is useful for microbiologic diagnosis, drainage, and relief of increased intracranial pressure.
A short course of corticosteroids may be useful in patients with brain abscess who have deteriorating neurologic status and increased intracranial pressure.
Cranial subdural empyema should be suspected in patients with headache, vomiting, fever, change in mental status, and rapid progression of focal neurologic signs.
Spinal epidural abscess may develop acutely or chronically, with symptoms and signs of focal vertebral pain, nerve root pain, motor or sensory defects, and paralysis; the transition to paralysis may be rapid, indicating the need for emergent evaluation, diagnosis, and treatment.
Surgical therapy is essential for the management of subdural empyema because antibiotics do not reliably sterilize these lesions.
Rapid surgical decompression should be performed in patients with spinal epidural abscess who have increasing neurologic deficit, persistent severe pain, or increasing temperature or peripheral white blood cell count.
Lateral gaze palsy may be an early clue to the diagnosis of cavernous sinus thrombosis because the abducens nerve is the only cranial nerve traversing the interior of the cavernous sinus.
The noninvasive diagnostic procedure of choice for suppurative intracranial thrombophlebitis is magnetic resonance imaging, which can differentiate between thrombus and normally flowing blood.
Bacterial infections of the central nervous system (CNS) are frequently devastating. The brain possesses several defense mechanisms (eg, intact cranium and blood-brain barrier) to prevent entry of bacterial species, but once microorganisms have gained entry to the CNS, host defense mechanisms are inadequate to control the infection. Antimicrobial therapy is limited by the poor penetration of many agents into the CNS and by the ability of antibiotics to induce inflammation in the CNS via their bacteriolytic action, thereby contributing to brain damage. We review meningitis, brain abscess, subdural empyema, epidural abscess, and suppurative intracranial thrombophlebitis, with an emphasis on recent developments in diagnosis and therapy as they pertain to the care of the critically ill patient.
MENINGITIS
The rates of morbidity and mortality from bacterial meningitis remain unacceptably high despite the availability of effective antimicrobial therapy. In a surveillance study of all cases of bacterial meningitis in 27 states of the United States from 1978 through 1981, the overall annual attack rate of bacterial meningitis was approximately 3.0 cases per 100,000 population, although there was variability according to geographic area, sex, and race;1 incidences for the various meningeal pathogens are listed in Table 71-1. Bacterial meningitis is also a significant problem in hospitalized patients. In a review of 493 episodes of bacterial meningitis in adults 16 years or older from the Massachusetts General Hospital from 1962 through 1988, 40% of cases were nosocomial in origin, and these episodes carried a high mortality rate (35% for single episodes of nosocomial meningitis).2 With the introduction of Haemophilus influenzae type b conjugate vaccines in the United States and elsewhere, dramatic declines in the incidence of invasive H influenzae type b disease have been reported.3 In a study that evaluated the epidemiology of bacterial meningitis in the United States during 1995 in laboratories serving all the acute care hospitals in 22 counties in four states (Georgia, Tennessee, Maryland, and California),4 the incidence of bacterial meningitis decreased dramatically as a result of the vaccine-related decline in meningitis caused by H influenzae type b (see Table 71-1). In another CDC surveillance study performed from 1998 to 2003, there was also a significant decline in the incidence of cases of pneumococcal meningitis,5 likely a result of introduction of the heptavalent pneumococcal conjugate vaccine in 2000. Implementation of the use of conjugate vaccines has dramatically changed the incidence of bacterial meningitis, such that it now occurs more commonly among adults.6
Etiology of Bacterial Meningitis in the United States
| Percent of Total | |||
|---|---|---|---|
| Organism | 1978-1981 | 1995 | 1998-2003 |
| Haemophilus influenzae | 48 | 7 | 7 |
| Neisseria meningitidis | 20 | 25 | 14 |
| Streptococcus pneumoniae | 13 | 47 | 58 |
| Streptococcus agalactiae | 3 | 12 | 18 |
| Listeria monocytogenes | 2 | 8 | 3 |
| Othera | 8 | — | — |
| Unknown | 6 | — | — |
Before the development of effective vaccines against it, H influenzae type b was isolated in almost half of all cases of bacterial meningitis in the United States, but this microorganism currently accounts for only 7% of cases.4,5 About 40% to 60% of cases were seen in children ages 2 months to 6 years; of these, 90% were due to capsular type b strains. Disease is most likely initiated after nasopharyngeal acquisition of a virulent organism with subsequent systemic invasion. Haemophilus influenzae is unusual after age 6 years; isolation of the organism in this older group should suggest the possible presence of certain predisposing factors, including sinusitis, otitis media, epiglottitis, pneumonia, head trauma with a cerebrospinal fluid (CSF) leak, diabetes mellitus, alcoholism, splenectomy or asplenic states, and immune deficiency (eg, hypogammaglobulinemia).7 In a prospective evaluation of adult patients with community-acquired bacterial meningitis in the Netherlands, H influenzae accounted for 2% of culture-proven cases.8
Meningitis due to Neisseria meningitidis is most often found in children and young adults and may occur in epidemics, although more than 98% of cases are sporadic.9 Nasopharyngeal carriage of virulent organisms accounts for initiation of infection. In the United States, 32% of cases are caused by serogroup B, 32% by serogroup C, and 24% by serogroup Y.6 Infection is more likely in persons who have deficiencies in the terminal complement components (C5, C6, C7, C8, and perhaps C9), the so-called membrane attack complex; the incidence of neisserial infections is more than 8000-fold greater in this group than among other persons.10 An increased risk has also been observed in patients with dysfunctional properdin, suggesting a potential role for the alternative complement pathway in resistance against meningococci. It has been suggested that a screening test for complement function (ie, CH50) should be performed for patients who have invasive meningococcal infections,11 with consideration for direct assessment of terminal complement components and properdin proteins; this approach should be considered in patients with recurrent neisserial infection.
Pneumococcal meningitis is observed most frequently in adults (>30 years) and is often associated with distant foci of infection, such as pneumonia, otitis media, mastoiditis, sinusitis, and endocarditis; this organism currently accounts for 58% of cases of bacterial meningitis in the United States.5 Serious pneumococcal infections may be observed in persons with predisposing conditions, such as splenectomy or asplenic states, multiple myeloma, hypogammaglobulinemia, and alcoholism. In children with cochlear implants with positioners who are beyond 24 months after implantation, there is an increased incidence of bacterial meningitis with most cases caused by S pneumoniae.12Streptococcus pneumoniae is the most common meningeal isolate in head trauma patients who have basilar skull fracture with subsequent CSF leakage13; remote head injury and CSF leak are important predisposing factors for recurrent bacterial meningitis.14 In one study of 352 episodes of community-acquired pneumococcal meningitis in adults, 70% of episodes were associated with an underlying disorder and the overall in-hospital mortality rate was 30%.15 Rates of pneumococcal meningitis have been reported to decrease among children and adults since introduction of the heptavalent pneumococcal conjugate vaccine,16 although there has been an increase in meningitis caused by nonvaccine serotypes. A 13-valent pneumococcal conjugate vaccine, which covers some of these additional serotypes, has recently been licensed and recommended for use.17
Listeria monocytogenes accounts for only about 3% of all cases of bacterial meningitis in the United States, but carries a high mortality rate.4,5 Infection with Listeria is more likely in neonates, the elderly, alcoholics, cancer patients, and immunosuppressed adults (eg, renal transplant patients).18,19 Cases have also been reported in patients receiving antitumor necrosis factor α agents (eg, infliximab and etanercept). Listeria meningitis is found infrequently in patients with human immunodeficiency virus infection, despite its increased incidence in patients with deficiencies in cell-mediated immunity. However, up to 30% of adults and 54% of children and young adults with listeriosis have no apparent underlying condition. Listeriosis has been associated with several food-borne outbreaks involving contaminated cole slaw, milk, cheese, and processed meats. In recent years, the incidence of invasive disease caused by L monocytogenes has been decreasing, likely as a result a decrease in organism contamination in ready-to-eat food.20
Meningitis due to aerobic gram-negative bacilli is observed in specific clinical situations.13,21Escherichia coli is isolated in 30% to 50% of infants younger than 2 months with bacterial meningitis. Klebsiella species, E coli, and Pseudomonas aeruginosa may be isolated in patients who have had head trauma or neurosurgical procedures, in the elderly, in immunosuppressed patients, and in patients with gram-negative bacteremia. Some cases have been associated with disseminated strongyloidiasis in the hyperinfection syndrome, in which meningitis is caused by enteric bacteria due to seeding of the meninges during persistent bacteremia associated with migration with infected larvae; alternatively, larvae may carry enteric organisms on their surfaces or within their own gastrointestinal tracts as they exit the gut and invade the meninges.
Specific clinical situations also predispose to the development of meningitis due to staphylococcal species. Staphylococcus epidermidis is the most common cause of meningitis in persons with CSF shunts.13 Meningitis due to Staphylococcus aureus is frequently found (when compared with other pathogens) after head trauma or soon after neurosurgery, or in those with infective endocarditis or paraspinal infection.22 Underlying diseases among persons with no prior CNS disease who develop S aureus meningitis include diabetes mellitus, alcoholism, chronic renal failure requiring hemodialysis, and malignancies. Conditions that increase S aureus nasal carriage rates (eg, injection drug abuse, insulin-requiring diabetes, and hemodialysis) may also predispose to staphylococcal infection of the CNS.
Group B streptococcus (Streptococcus agalactiae) is a common cause of meningitis in neonates21,23; 66% of all cases have been reported during the first 3 months of life. The risk of transmission from the mother to her infant is increased when the inoculum of organisms and number of sites of maternal colonization are large; horizontal transmission has also been documented from the hands of nursery personnel to the infant. Risk factors for group B streptococcal meningitis in adults include age older than 60 years, diabetes mellitus, parturient status in women, cardiac disease, collagen vascular disease, malignancy, alcoholism, hepatic failure, renal failure, and corticosteroid therapy. No underlying illnesses were found in 43% of patients in one review.24
The classic clinical presentation in adults with bacterial meningitis includes fever, headache, meningismus, and signs of cerebral dysfunction.21 In one review of 493 cases of acute bacterial meningitis in adults,2 the triad of fever, nuchal rigidity, and change in mental status was only found in two-thirds of patients, but all had at least one of these findings. In another review of 696 cases of community-acquired bacterial meningitis,25 the triad of fever, neck stiffness, and altered mental status was found in only 44% of episodes, although almost all patients (95%) presented with at least two of four symptoms (headache, fever, stiff neck, altered mental status). Also seen are nausea, vomiting, rigors, profuse sweating, weakness, and myalgias. The meningismus may be subtle or marked or accompanied (rarely) by the Kernig and/or Brudzinski signs. The Kernig sign is elicited by flexing the thigh on the abdomen with the knee flexed; the leg is then passively extended, and, if there is meningeal inflammation, the patient resists leg extension. The Brudzinski sign is present when passive flexion of the neck leads to flexion of the hips and knees. However, these signs are elicited in fewer than 5% of cases of bacterial meningitis in adults,26 indicating that they do not accurately distinguish patients with meningitis from those without meningitis. Cerebral dysfunction is manifested by confusion, delirium, or a declining level of consciousness ranging from lethargy to coma. Cranial nerve palsies (especially involving cranial nerves III, IV, VI, and VII) and focal cerebral signs are uncommon (10%-20% of cases). Seizures occur in about 30% of all cases. Papilledema is rare (<5%) and should suggest an alternate diagnosis, such as an intracranial mass lesion. To further characterize the accuracy and precision of the clinical examination in adult patients with acute meningitis, data from 845 episodes were reviewed and demonstrated that individual items of the clinical history (ie, headache, nausea, and vomiting) had a low accuracy for the diagnosis of acute meningitis; on review of the physical examination, the absence of fever, neck stiffness, and altered mental status effectively eliminated the likelihood of acute meningitis (sensitivity 99%-100% for the presence of one of these findings in diagnosis).27 However, despite these results, physicians should have a low threshold for performing a lumbar puncture in patients with suspected bacterial meningitis. Late in the disease, patients may develop signs of increased intracranial pressure, including coma, hypertension, bradycardia, and third-nerve palsy; these findings are ominous prognostic signs.
Certain symptoms and signs may suggest an etiologic diagnosis in patients with bacterial meningitis.21 Persons with meningococcemia present with a prominent rash, principally on the extremities (∼50% of cases). Early in the disease course, the rash may be erythematous and macular, but it quickly evolves into a petechial phase, with further coalescence into a purpuric form. The rash often matures rapidly, with new petechial lesions appearing during the physical examination. A petechial, purpuric, or ecchymotic rash may also be seen in other forms of meningitis (ie, those due to echovirus type 9, Acinetobacter species, S aureus, and, rarely, S pneumoniae or H influenzae), in Rocky Mountain spotted fever or S aureus endocarditis, and in overwhelming sepsis (due to S pneumoniae or H influenzae) in splenectomized patients. An additional suppurative focus of infection (eg, otitis media, sinusitis, or pneumonia) is present in 30% of patients with pneumococcal or H influenzae meningitis but is rarely found in meningococcal meningitis. Meningitis due to S pneumoniae is relatively likely after head trauma in persons who have basilar skull fractures in which a dural fistula is produced between the subarachnoid space and the nasal cavity, paranasal sinuses, or middle ear.10 These persons commonly present with rhinorrhea or otorrhea due to a CSF leak; a persistent defect is a common explanation for recurrent bacterial meningitis. Patients with Listeria meningitis have an increased tendency to have seizures and focal neurologic deficits early in infection and may present with other features consistent with rhombencephalitis (ie, ataxia, cranial nerve palsies, or nystagmus).18,19
Certain subgroups of patients may not manifest the classic signs and symptoms of bacterial meningitis.21 Usually in a neonate there is no meningismus or fever, and the only clinical clues to meningitis are listlessness, high-pitched crying, fretfulness, refusal to feed, irritability, vomiting, diarrhea, respiratory distress, seizures, or bulging fontanelle.28 Elderly patients, especially those with underlying conditions such as diabetes mellitus or cardiopulmonary disease, may present with insidious disease manifested only by lethargy or obtundation, variable signs of meningeal irritation, and no fever. In this subgroup, altered mental status should not be ascribed to other causes until bacterial meningitis has been excluded by CSF examination. A patient after neurosurgery or a patient who has undergone head trauma also presents a unique clinical situation because these patients already have many of the symptoms and signs of meningitis from their underlying disease processes13; clinical features are variable, but most commonly include fever and an altered level of consciousness. One must have a low threshold for CSF examination in these patients should they develop any clinical deterioration.
The diagnosis of bacterial meningitis rests on the CSF examination.21,29,30 The opening pressure is elevated in virtually all cases; values above 600 mm H2O suggest cerebral edema, the presence of intracranial suppurative foci, or communicating hydrocephalus. The fluid may be cloudy or turbid if the white blood cell count is elevated (>200/µL). If the lumbar puncture is traumatic, the CSF may appear bloody initially, but it should clear as flow continues. Xanthochromia, a pale-pink to yellow-orange color of the supernatant of centrifuged CSF, is found in patients with subarachnoid hemorrhage, usually within 2 hours after hemorrhage.
The CSF white cell count is usually elevated in untreated bacterial meningitis, ranging from 100 to at least 10,000 per microliter, with a predominance of neutrophils. About 10% of patients present with a lymphocytic predominance (>50%) in CSF. Some patients (especially those with septic shock and systemic complications) have a very low CSF white cell count (0-20/µL) despite high bacterial concentrations in CSF; these patients have a poor prognosis. Therefore, a Gram stain and culture should be performed on all CSF specimens, even those with a normal cell count. A CSF glucose concentration of less than 40 mg/dL is found in about 60% of patients with bacterial meningitis, and a CSF:serum glucose ratio of less than 0.31 is observed in 70% of cases. The CSF glucose level must always be compared with a simultaneous serum glucose concentration. The CSF protein concentration is elevated in virtually all cases of bacterial meningitis, presumably because of disruption of the blood-brain barrier.
CSF examination by Gram stain permits a rapid, accurate identification in 60% to 90% of cases of bacterial meningitis; the likelihood of detecting the organism by Gram stain correlates with the specific bacterial pathogen and the concentration of bacteria in CSF. False-positive findings may occur as a result of contamination in the collection of tubes or during staining. Cultures of CSF are positive in 70% to 80% of cases. These percentages may be lower in patients who have received prior antimicrobial therapy.
Several rapid diagnostic tests have been developed to aid in the diagnosis of bacterial meningitis.21,29,30 Latex agglutination tests are rapid and sensitive, although the routine use of CSF bacterial antigen tests for the etiologic diagnosis of bacterial meningitis has been questioned; positive results have not modified therapy and false-positive and false-negative results may occur. Measurement of serum C-reactive protein or procalcitonin may also be useful in discriminating between bacterial and viral meningitis because elevated serum concentrations of these proteins (≥20 mg/L and ≥0.5ng/mL, respectively) have been observed in patients with acute meningitis. In patients with acute meningitis in whom the CSF Gram stain is negative, serum concentrations of C-reactive protein or procalcitonin that are normal or below the limit of detection have a high negative predictive value in the diagnosis of bacterial meningitis. An immunochromatographic test for the detection of S pneumoniae in CSF was found to have an overall sensitivity of 95% to 100% for the diagnosis of pneumococcal meningitis,31 although more studies are needed. Nucleic acid amplification tests, such as polymerase chain reaction (PCR), have been used to amplify DNA from patients with bacterial meningitis. Several studies have shown that broad-based PCR has excellent sensitivity, specificity, and positive and negative predictive values in the diagnosis of bacterial meningitis.21,29,30 The sensitivity and specificity of PCR for the diagnosis of pneumococcal meningitis are 92% to 100% and 100%, respectively.31 A recently developed nucleic acid amplification test, loop-mediated isothermal amplification, is a promising tool especially in resource-poor settings.28 Further refinements in PCR may render it useful in the diagnosis of bacterial meningitis when the CSF Gram stain and cultures are negative; PCR may also prove to be beneficial to detect in vitro susceptibility of meningeal pathogens to specific antimicrobial agents.
Neuroimaging techniques have little role in the diagnosis of acute bacterial meningitis, except to rule out the presence of other pathologic conditions or to identify a parameningeal source of infection.21 However, computed tomography (CT) or magnetic resonance imaging (MRI) may be useful in patients who have a persisting fever several days after initiation of antimicrobial therapy, prolonged obtundation or coma, new or recurrent seizure activity, signs of increased intracranial pressure, or focal neurologic deficits. MRI is better than CT for evaluation of subdural effusions, cortical infarctions, and cerebritis, although it is more difficult to obtain an MRI in a critically ill patient, which limits its usefulness in many patients with meningitis.
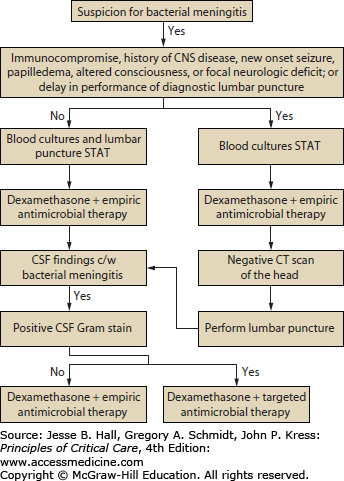
Antimicrobial Therapy: The initial approach to the patient with suspected bacterial meningitis is to perform a lumbar puncture to determine whether the CSF findings are consistent with that diagnosis (Fig. 71-1).21,29,30 Patients should receive empirical antimicrobial therapy based on their age and underlying disease status (Table 71-2),21,29,32 if no etiologic agent is identified by Gram stain; if the Gram stain is positive, targeted antimicrobial therapy can be initiated (Table 71-3). In patients with certain clinical features at presentation (see below), CT should be performed immediately to exclude an intracranial mass lesion because lumbar puncture is relatively contraindicated in that setting. However, obtaining a CT scan generally entails some delay, so empirical antimicrobial therapy should be started immediately, before the CT scan and lumbar puncture are done and after obtaining blood cultures, because of the high mortality rate in patients with bacterial meningitis in whom antimicrobial therapy is delayed. Although many clinicians routinely perform CT before lumbar puncture, this is probably not necessary in most patients. In a recent retrospective study of 301 adults with suspected meningitis,33 the clinical features at baseline that were associated with an abnormal finding on CT of the head were an age of at least 60 years, immunocompromise, a history of CNS disease, a history of seizure within 1 week before presentation, and the following neurologic abnormalities: an abnormal level of consciousness, an inability to answer two consecutive questions correctly or to follow two consecutive commands, gaze palsy, abnormal visual fields, facial palsy, arm drift, leg drift, and abnormal language. These results need to be validated but are a reasonable guide in determining which patients require CT before lumbar puncture. It is reasonable to proceed with lumbar puncture without performing a CT scan in patients who do not have new-onset seizures, an immunocompromised state, signs that are suspicious for space-occupying lesions (eg, papilledema or focal neurologic findings), or moderate or severe impairment of consciousness.29 However, despite these guidelines, the decision to perform a lumbar puncture without first doing a CT scan must be individualized. In addition, a normal CT scan does not always mean that performance of a lumbar puncture is safe; clinical signs that suggest the need to delay lumbar puncture include those of impending herniation (eg, deteriorating level of consciousness, particularly a Glasgow Coma Scale score ≤11; brain stem signs such as pupillary changes, posturing, or irregular respirations; or a very recent seizure).34
FIGURE 71-1
Management algorithm for adults with suspected bacterial meningitis. See text and tables for specific recommendations for empirical (Table 71-2) and targeted therapy (Table 71-3). (Reproduced with permission from Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. November 1, 2004;39(9):1267-1284.)
Targeted Antimicrobial Therapy for Acute Bacterial Meningitis with Presumptive Pathogen Identification by Gram Stain
| Microorganism | Recommended Therapy |
|---|---|
| Streptococcus pneumoniae | Vancomycin plus a third-generation cephalosporina,b |
| Neisseria meningitidis | Third-generation cephalosporina |
| Haemophilus influenzae | Third-generation cephalosporina |
| Streptococcus agalactiae | Ampicillinc or penicillin Gc |
| Listeria monocytogenes | Ampicillinc or penicillin Gc |
Our choices for empirical antibiotic therapy in patients with presumed bacterial meningitis, based on age, are presented in Table 71-2.21,29,32 For neonates younger than 1 month, the most likely infecting organisms are E coli, S agalactiae, and L monocytogenes; for those ages 1 to 23 months, infection may be due to S pneumoniae, N meningitidis, S agalactiae, E coli, or H influenzae. From age 2 to 50 years, most cases of meningitis are due to N meningitidis and S pneumoniae. In older adults (≥50 years), the meningococcus and the pneumococcus are possible causes, as are L monocytogenes and gram-negative bacilli. For all patients in whom S pneumoniae is a possible causative pathogen (essentially all patients ≥1 month of age), vancomycin should be added to empirical therapeutic regimens because highly penicillin- or cephalosporin-resistant strains of S pneumoniae may be likely (see below). One other situation deserves comment—in patients after neurosurgery or patients with CSF shunts or foreign bodies, likely infecting organisms include staphylococci (S epidermidis or S aureus), diphtheroids (including Propionibacterium acnes), and gram-negative bacilli (including P aeruginosa).13,29 Antimicrobial therapy in these situations should consist of vancomycin plus either ceftazidime, cefepime, or meropenem pending culture results.
Once an infecting microorganism has been isolated, antimicrobial therapy can be modified for optimal treatment.21,29,32 Our antibiotics of choice are listed in Table 71-4. Dosages for adults are listed in Table 71-5. For bacterial meningitis due to susceptible strains of S pneumoniae or N meningitidis, penicillin G and ampicillin are equally efficacious. Although in past years pneumococci remained uniformly susceptible to penicillin (minimal inhibitory concentration ≤0.06µg/mL), worldwide reports have now documented resistant strains of pneumococci. In view of these recent trends, and because sufficient CSF concentrations of penicillin are difficult to achieve with standard high parenteral doses (initial CSF concentrations of ∼1µg/mL), penicillin can never be recommended as empirical antimicrobial therapy when S pneumoniae is considered a likely infecting pathogen. Further, susceptibility testing must be performed on all CSF isolates. For strains that are resistant to penicillin (MIC ≥0.12µg/mL, but sensitive to a third-generation cephalosporin (MIC <1µg/mL), cefotaxime or ceftriaxone should be used; for strains resistant to penicillin and third-generation cephalosporins, vancomycin in combination with a third-generation cephalosporin is the antimicrobial regimen of choice because vancomycin used alone, especially when combined with adjunctive dexamethasone, may not be optimal therapy for patients with pneumococcal meningitis. This combination should be continued pending results of susceptibility testing. Adequate CSF concentrations of vancomycin, however, may be attained even when patients are receiving adjunctive dexamethasone as long as appropriate dosages of vancomycin are administered; in one study of 14 patients,35 administration of intravenous vancomycin (15 mg/kg loading dose, followed by a continuous infusion of 60 mg/kg per day) led to mean CSF concentrations of 7.2µg/mL. Some investigators have recommended the addition of rifampin (if the organism is susceptible) to the combination of vancomycin plus the third-generation cephalosporin for the treatment of meningitis caused by highly resistant pneumococcal strains,21,29,32 although there are no firm data to support this. Meropenem, a carbapenem antimicrobial agent, yields microbiologic and clinical outcomes similar to those of cefotaxime or ceftriaxone in the treatment of patients with bacterial meningitis. The newer fluoroquinolones (eg, moxifloxacin) have in vitro activity against resistant pneumococci and have shown activity in experimental animal models of resistant pneumococcal meningitis,21,29,32 but should not be used as first-line therapy in patients with bacterial meningitis, pending results of ongoing clinical trials. Trovafloxacin was shown to be therapeutically equivalent to ceftriaxone with or without vancomycin for the treatment of pediatric bacterial meningitis,36 although this agent is no longer used because of concerns of liver toxicity. The combination of moxifloxacin plus either vancomycin or a third-generation cephalosporin may emerge as a treatment option for patients with pneumococcal meningitis.
Antimicrobial Therapy of Bacterial Meningitis
| Organism | Antibiotic of Choice |
|---|---|
| Streptococcus pneumoniae | |
| Penicillin MIC ≤0.06µg/mL | Penicillin G or ampicillin |
| Penicillin MIC ≥0.12µg/mL | |
| Ceftriaxone or cefotaxime MIC <1.0µg/mL | Third-generation cephalosporina |
| Ceftriaxone or cefotaxime MIC ≥1.0µg/mL | Vancomycin plus a third-generation cephalosporina,d |
| Neisseria meningitidis | Penicillin G or ampicillin, or a third-generation cephalosporina |
| Haemophilus influenzae | |
| β-Lactamase negative | Ampicillin |
| β-Lactamase positive | Third-generation cephalosporina |
| Enterobacteriaceaeb | Third-generation cephalosporina |
| Pseudomonas aeruginosa | Ceftazidimec or cefepimec |
| Streptococcus agalactiae | Penicillin Gc or ampicillinc |
| Listeria monocytogenes | Ampicillinc or penicillin Gc |
| Staphylococcus aureus | |
| Methicillin sensitive | Nafcillin or oxacillin |
| Methicillin resistant | Vancomycin |
| Staphylococcus epidermidis | Vancomycind |
Recommended Doses of Antibiotics for Intracranial Infectionsin Adults With Normal Renal Function
| Antibiotic | Total Daily Dose in Adults (Dosing Interval) |
|---|---|
| Amikacina | 15 mg/kg (q8h) |
| Ampicillin | 12 g (q4h) |
| Aztreonam | 6-8 g (q6-8h) |
| Cefepime | 6 g (q8h) |
| Cefotaxime | 8-12 g (q4-6h) |
| Ceftazidime | 6 g (q8h) |
| Ceftriaxone | 4 g (q12-24h) |
| Ciprofloxacin | 800-1200 mg (q8-12h) |
| Gentamicin,a tobramycina | 5 mg/kg (q8h) |
| Meropenem | 6 g (q8h) |
| Metronidazole | 30 mg/kg (q6h) |
| Nafcillin, oxacillin | 9-12 g (q4h) |
| Rifampin | 600 mg (q24h) |
| Trimethoprim-sulfamethoxazole | 10-20 mg/kgb (q6-12h) |
| Vancomycinc | 30-60 mg/kg (q8-12h) |
Meningococcal strains that are relatively resistant to penicillin have also been reported from several areas (in particular Spain); however, most patients harboring these strains have recovered with standard penicillin therapy, so their clinical significance is unclear. In addition, in one study in Ontario, there was no association between invasive meningococcal disease in decreased susceptibility to penicillin and mortality, although there was a marked increase in mortality associated with infection caused by serogroups B and C.37 In the United States, approximately 3% of meningococcal strains have shown intermediate susceptibility to penicillin.21,29,32 Some authorities would treat meningococcal meningitis with a third-generation cephalosporin (cefotaxime or ceftriaxone) pending results of in vitro susceptibility testing.
Treatment of H influenzae type b meningitis has been hampered by the emergence of β-lactamase–producing strains of the organism, which accounted for approximately 25% to 33% of all isolates in the United States.21 Chloramphenicol resistance has also been reported in the United States (<1% of isolates) and Spain (≥50% of isolates). In addition, a study found chloramphenicol to be bacteriologically and clinically inferior to certain β-lactam antibiotics (ampicillin, ceftriaxone, and cefotaxime) in childhood bacterial meningitis, and most of these cases were due to H influenzae type b. From these findings and those of other studies, the third-generation cephalosporins (eg, cefotaxime and ceftriaxone) seem to be at least as effective as ampicillin plus chloramphenicol for therapy of H influenzae meningitis. Cefuroxime, a second-generation cephalosporin, has also been evaluated for therapy of H influenzae
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree