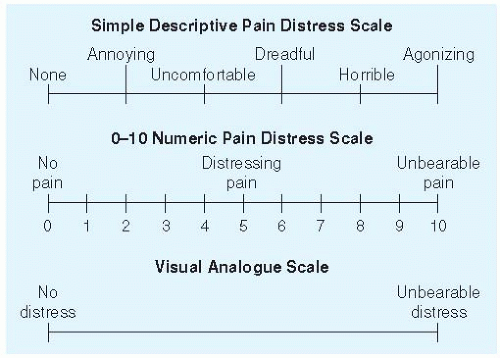
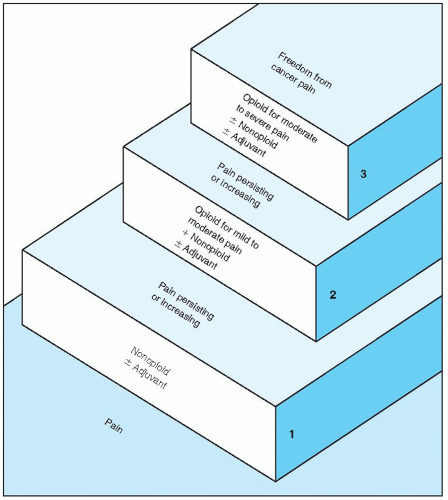
Nonopioid Analgesics |
Oxycodone |
Acetaminophen and salicylates |
|
Roxicodone, 5, 15, 30 mg |
|
Acetaminophen, up to 650 mg q4h or 975 mg q6h Aspirin, up to 650 mg q4h or 975 mg q6h |
|
OxyContin, 10, 20, 40, 80 mg Liquid, 5 mg/5 mL, 20 mg/mL |
Nonsteroidal Antiinflammatory Drugs |
Oxycodone with acetaminophen |
|
Ibuprofen (Motrin, Advil), 600 mg q6h |
|
Percocet, Roxicet, Tylox, Endocet, generic, 2.5, 5, 7.5, 10 mg |
|
Naproxen (Naprosyn, Aleve), 250-500 mg bid |
Oxycodone with aspirin |
|
Ketorolac (Toradol), 30 mg IM or IV q6h (up to 5 d) |
|
Percodan, 5 mg |
|
Celecoxib (Celebrex), 100-200 mg bid |
Oxymorphone |
Opioid Analgesics |
|
Opana IR, 5, 10 mg |
Morphine |
|
Opana ER, 5, 10, 20, 40 mg |
|
MSIR tablets or capsules, 15, 30 mg |
Levorphanol |
|
MSIR “soluble” tablets, 10, 15, 30 mg |
|
Levo-Dromoran, generic, 2 mg |
|
Roxanol (elixir), 10 mg/5 mL, 20 mg/5 mL, 100 mg/5 mL |
Methadone |
|
MS Contin, 15, 30, 60, 100, 200 mg |
|
Dolophine, generic, 5, 10 mg |
|
Oramorph SR, 15, 30, 60, 100 mg |
|
Dispertab, 40 mg |
|
Avinza, 30, 60, 90, 120 mg |
|
Liquid, 1 mg/mL, 10 mg/5 mL, 10 mg/mL |
|
Kadian, 20, 30, 50, 60, 80, 100, 200 mg |
Adjuvants |
Fentanyl |
Antidepressants |
|
Duragesic transdermal patches, 12.5, 25, 50, 75, 100 µg |
|
Desipramine 10-150 mg qhs |
|
Actiq transmucosal lozenges, 200, 400, 600, 800, 1,200, 1,600 µg |
|
Nortriptyline 10-150 mg qhs |
|
Fentora buccal tablets, 100, 200, 400, 600, 800 µg |
|
Venlafaxine (Effexor, Effexor XR), 75-225 mg/d |
Hydromorphone |
|
Dilaudid, generic, 2, 4, 8 mg; liquid 5 mg/5 mL |
Duloxetine (Cymbalta), 30-60 mg daily |
|
Suppository, 3 mg |
|
Anticonvulsants |
|
Exalgo extended release, 8, 12, 16 mg |
|
Carbamazepine (Tegretol), 100-800 mg bid |
Codeine |
|
Gabapentin (Neurontin), 100-400 mg tid |
|
Codeine sulfate tablets, 15, 30, 60 mg |
|
Pregabalin (Lyrica), 50-200 mg tid |
|
Elixir and solution, 15 mg/mL |
|
Phenytoin (Dilantin), 300-500 mg/d |
Codeine with acetaminophen |
Stimulants |
|
Tylenol No. 2, No. 3, No. 4 (15, 30, 60 mg, respectively) |
|
Dextroamphetamine (Dexedrine), 5-10 mg/d bid |
Codeine with aspirin |
|
Empirin No. 3, No. 4, 30, 60 mg, respectively |
|
Methylphenidate (Ritalin), 2.5-15.0 mg qd-bid |
Hydrocodone with acetaminophen |
|
Modafinil (Provigil), 200-400 mg daily |
|
Lortab, 2.5, 5, 7.5, 10 mg |
Corticosteroids |
|
Lorcet, 5, 7.5, 10 mg |
|
Dexamethasone (Decadron), 4-96 mg bid-qid |
|
Vicodin, 5, 7.5 mg |
|
Prednisone, 10-100 mg/d bid |
bid, twice daily; q4h, every 4 hours; q6h; every 6 hours; qd, daily; qhs, at bedtime; qid, four times a day; tid, three times a day; PO, orally; IM, intramuscularly.
Adapted from Jacox A, Carr DB, Payne R, et al. Management of cancer pain. Clinical practice guideline No. 9 (AHCPR publication No. 94-0592). Rockville, MD: Agency for Health Care Policy and Research, U.S. Department of Health and Human Services, Public Health Service, March 1994; and Fowler B, Lynch M, Abrahm J. Pain management tables and guidelines. Boston, MA: Dana Farber Cancer Institute Pain and Palliative Care Program, 2004. |
|