Arterial Blood Gas (ABG) Sampling:
Links: Procedure | A-a Gradient | Acid-Base Physiology & Interpretation |
Reason: to asses if adequate gas exchange.
Abbreviations: PB = barometric pressure (mm Hg). FIO2 = inspired oxygen fraction (0.21 = room air). PaCO2 = partial pressure of carbon dioxide in arterial blood (mm Hg). PACO2 = partial pressure of carbon dioxide in alveolar gas (mm Hg). PaO2 = partial pressure of oxygen in arterial blood (mm Hg). PAO2 = partial pressure of oxygen in alveolar gas (mm Hg).
Normal values at sea level room air (FIO2 = 0.21):
PaO2 –> 80-95 mmHg. The oxygenation.
If normal but cyanotic with decr sat think met-Hb.
If sat high for the PaO2 think cyanide or carboxy-Hb.
PaCO2 –> 35-45 mmHg. The ventilation.
SaO2 –> 96-99%.
pH –> 7.40 +0.02.
HCO3 –> 22-28 mEq/L
Base excess or deficit: -3 to +3 mEq/L.
Values should not change with age except decr PaO2.
For age 40-90yo, PaO2 = 108.75 – 0.39 X age. (Or ~100-1/3 age)
Mixed venous O2 tension (PVO2): 35-50mmHg.
Mixed venous O2 concentration (CVO2): 12-15ml/dL. Or 70-75% sat.
Arterial O2 concentration (CaO2): 17-20 ml/dL.
Arteriovenous O2 difference = C(a-v)O2: 4-5ml/dL.
ABG Procedure: Best to use radial > femoral or brachial artery. Contra: +Allen test, ESRD (may need shunt or fistula site), bleeding d/o. Equipment: 3-5ml plastic syringe X2, one with 2ml of 1% Lido. Two 25g, ½” needles, ice, ETOH or Iodine, drape, 1ml of Heparin 10,000 U/ml, sterile gloves, 2X2’s, towel, tape.
Step #1: Check Allen Test: asses patency of collateral circulation. Occlude both radial & ulnar arteries with firm pressure at distal forearm. Elevate arm, pt make a few fists to drain blood. Release pressure on ulnar side, should pink up hand in <6sec.
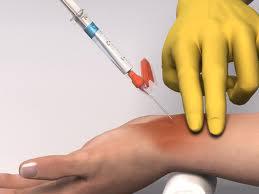 Step #2: Place rolled towel under hyperextended wrist, tape to back board. Palpate radial artery as it lies between styloid process of radius & flexor carpi ulnaris tendon. PMI should be just proximal to transverse wrist crease.
Step #2: Place rolled towel under hyperextended wrist, tape to back board. Palpate radial artery as it lies between styloid process of radius & flexor carpi ulnaris tendon. PMI should be just proximal to transverse wrist crease.
Step #3: Don sterile gloves. Wet the syringe with Heparin and evacuate the extra (extra heparin left in syringe –> decr pH).
Prep area with Betadine or ETOH, then give local anesthetic (optional), infiltrate skin and then down to periosteum or radius on either side of the artery with 1%Lido (may decr vasospasm).
Step #4: Tell pt to expect some discomfort but to keep as still as possible. Relocate PMI with nondominant hand as face the pt. Hold syringe like-a-dart, insert bevel up into skin at 60 deg pointing proximally. Slowly advance until 1-2ml of pulsatile blood fills syringe passively.
If no blood advance until hit bone, then slowly pull back to skin (may have pierced the artery), once needle is just beneath skin, repalpate artery and redirect.
Step #5: withdraw needle, apply firm pressure to area for 5-10 min. Remove all air bubbles (affects PO2) from syringe, remove needle and apply rubber stopper. Label and place on ice or send to lab ASAP (within 20min) as WBC’s metabolically active. Can use elastic ace wrap and gauze pad to apply pressure, best not to let pt do it, can use an assistant. Acid-Base Physiology & Interpretation |
Complications: Pain, infection, local bleeding, venous or air contamination. Distal ischemia if over traumatize the artery (rotate sites, avoid brachial artery). AV fistula or false aneurysm (from over sampling same site)
Re-check pt in 15-20 min to r/o wrist hematoma and adequate perfusion. If unable to use radial artery due to +Allen, or local infection.
Brachial Artery: use 22g 1.5”. Consult a vascular surgeon ASAP if get transient spasm, occlusion or clotting of brachial artery. Distal embolization with blood or cholesterol emboli, ischemia or gangrene of hand or forearm.
Femoral Artery: Commonly use is hypovolemia or shock. Can use blind sampling of the best estimated position of the femoral artery. Position of vascular structure:
Lat –> Med: NAVAL: Nerve, artery, vein, empty space, lymphatics.
If no pulse palpable –> divide the distance between ASIS & Pubic tubercle in thirds. The artery lies at a point where the inner 1/3 and middle 1/3 meets.
Alveolar-arterial O2 Gradient (A-a)
Useful in determining the source of hypoxemia when pt is on room air.
PAO2 = 150 – (PaCO2 x 1.25 [= 5/4 or 1 + 1/4]) = O2 gradient = PAO2 – PaO2. Normal = 5-20 mmHg.
• Normal is <10-20 mmHg breathing room air at sea level for any age, or 10-60 on 100% O2.
• If elevated, it indicates respiratory dz that is interfering with gas exchange. If on O2, can remove and the O2 sat will equilibrate in 5 min, as hard to interpret unless on RA or 100%. = 148 – 1.2(PaCO2) – PaO2. 148 = 713 X FiO2. = PAO2 – PaO2.
• Any incr A-a gradient means an abnormal lung.
Short-cut estimate: 140 – (PaCO2 + PaO2)
• A-a Gradient gauges effectiveness of oxygenation.
• Normal on RA is 5-20 mmHg.
• If A-a gradient is < 20 mmHg, then there is NOT a problem in the alveolar-capillary unit…due to low external O2 or hypoventilation.
• If A-a gradient is > 20 mmHg, then there IS a problem in the alveolar-capillary unit.
• A-a Gradient cannot be negative. If it calculates to a negative number, then the ABG values are factitious (cross out that test exam answer!).
• The most important first step in determining the cause of hypoxemia at rest.
A-a Gradient and the 5 Mechanisms of Hypoxemia:
• Low external O2: A-a Gradient normal.
• Hypoventilation: A-a Gradient normal.
• Diffusion defect: A-a Gradient widened. Note: a diffusion defect is almost never the cause of hypoxemia at rest.
• V/Q mismatch: A-a Gradient widened.
• True Shunt: A-a Gradient widened.
• • • Since can generally throw out low FiO2 and Diffusion defect, the causes of Hypoxemia comes down to 3 things that can be delineated by the A-a gradient and response to low flow FI02.
1. Hypoventilation: Normal A-a Gradient. Pa02 improves with O2. Tx with ventilation.
2. V/Q Mismatch: Abnormal A-a Gradient. Pa02 improves with O2. Tx with O2.
3. True Shunt: Abnormal A-a Gradient. No change Pa02 with O2. Treat with positive pressure +/- PEEP.
Upper Limit of Normal a-A gradient in mm Hg for age:
As a quick calculation, a normal A-a gradient is less than the (patient’s age divided by 4) + 4. Thus, a 40 year old should have an A-a gradient less than 14.
Adolescents = <10 mm Hg.
18-60yo–> 10-20 mmHg.
60-69–> 25.
>= 70yo–> 30.
Example: Pt’s labs show: pH 7.47. PC02 32 mmHg. P02 92 mmHg
PAO2 = 150 – (32 x 1.25) = 150 – 40 = 110
PAO2 – PaO2 = 110 – 92 = 18 = A-a = Normal.
• (FIO2 X 713) – 1.25(PaCO2) – PaO2.
• (PAO2 is calc = all the O2 in the alveoli available. PaO2 is measured on the ABG and equals the the amount of O2 dissolved in the plasma)
Normal = 4 + [(age-20) x .3]
***Ref: (Acid base d/o’s, The Principles and Practice of Nephrology, 2005, Mosby) (Anion gap, Kidney Int 1985;27:472) (Postgrad Med 2000;107:3) (Acid-base. Arch IM 1992;152:1625-29) (Medicine 1980;59:161-187) (Acid-base d/o’s: classification and management strategies. Am Fam Physician. 1995;52:584-90) (The cellular basis of metabolic alkalosis. Kidney Int. 1996;49:906-17) (Metabolic acidosis with extreme elevation of anion gap: case report and literature review. Am J Med Sci. 1999;317:38-49) (An approach to clinical acid-base problem solving. Compr Ther. 1998;24:553-9) (Protection of acid-base balance by pH regulation of acid production. NEJM 1998;339:12) (Arch IM 1992;152;1625-29) (The urine anion gap. Am J Med 1986;292:198-202)
Acute Respiratory Distress Syndrome (ARDS):
Links: Acute Lung Injury | Etiology | S/s | CXR | Dx | Tx | See Shock |
Acute respiratory failure following a systemic or pulmonary insult. Edema from increased permeability. 1/3 of pt’s get as a complication of sepsis, a bad prognostic indicator that occurs in 35% of cases. Pro-inflammatory cytokines (TNF, IL-1)  released from stimulated lymphocytes and macrophages appear to be pivotal in lung injury –> damage to capillary endothelial cells and alveolar epithelial cells (type I pneumocytes). Mortality from acute respiratory distress syndrome (ARDS) continues to hover around 40-45%, as it has since 1994 when a consensus definition for the syndrome was published according to a comprehensive literature review and meta-analysis (Am J Respir Crit Care Med 2009;179:220-227)…..They speculate that the failure to improve ARDS outcomes reflects the dearth of effective treatments and variable adherence to guidelines that recommend low tidal volume, early aggressive resuscitation, and appropriate antibiotic use and sedation protocols.
released from stimulated lymphocytes and macrophages appear to be pivotal in lung injury –> damage to capillary endothelial cells and alveolar epithelial cells (type I pneumocytes). Mortality from acute respiratory distress syndrome (ARDS) continues to hover around 40-45%, as it has since 1994 when a consensus definition for the syndrome was published according to a comprehensive literature review and meta-analysis (Am J Respir Crit Care Med 2009;179:220-227)…..They speculate that the failure to improve ARDS outcomes reflects the dearth of effective treatments and variable adherence to guidelines that recommend low tidal volume, early aggressive resuscitation, and appropriate antibiotic use and sedation protocols.
Acute Lung Injury (ALI): less severe than ARDS, same definition except PaO2: FiO2 <300 mmHg (Vs <200). The most common predisposing factors were severe sepsis (with or without a pulmonary source), severe trauma, and aspiration…the in-hospital mortality is 39% with only 34% of survivors going directly home after the hospitalization (most went to skilled nursing or rehabilitation facilities) (Incidence and outcomes of acute lung injury. NEJM 2005;353:1685-93)….the authors estimate that 75,000 people in the U.S. die from acute lung injury each year and most survivors suffer substantial physical, emotional, and cognitive impairment beyond the acute hospitalization. Conservative fluid management after acute lung injury is associated with improved oxygenation, number of ventilator-free days, and central nervous system function (NEJM. Posted online May 21, 2006) (mean 7-day fluid balance was -136 mL in the conservative-strategy group Vs +6992 mL in the liberal-strategy group, mortality rates were 25.5% Vs 28.4%) (more furosemide and fewer transfusions)….. editorialist notes that these protocols were implemented an average of 43 hours after pt’s were admitted to the ICU, when most pt’s already had optimized hemodynamics. ALI is a serious complication that occurs early in the course of septic shock, and delayed treatment goal-directed resuscitation, recent chemotherapy, and delayed antibiotic administration were risk factors for acute lung injury in an observational cohort study with 160 consecutive adult patients with septic shock (Crit Care Med 2008;36:1518)…..Hospital mortality was almost three times higher in patients with ALI than in those without (51% vs. 18%).
Updated “Berlin Definition” of ARDS: (JAMA. 2012;1-8:doi:10.1001/jama.2012.5669). It takes into account timing of symptoms, chest imaging, origin of edema, and oxygenation, with three mutually exclusive categories of ARDS based on severity of hypoxemia.
Mild (200 mm Hg < PaO2/FIO2 = 300 mm Hg), moderate (100 mm Hg < PaO2/FIO2 = 200 mm Hg), and severe (PaO2/FIO2 = 100 mm Hg) and 4 ancillary variables for severe ARDS: radiographic severity, respiratory system compliance (=40 mL/cm H2O), positive end-expiratory pressure (=10 cm H2O), and corrected expired volume per minute (=10 L/min). Using the Berlin Definition, stages of mild, moderate, and severe ARDS were associated with increased mortality (27%; 95% CI, 24%-30%; 32%; 95% CI, 29%-34%; and 45%; 95% CI, 42%-48%, respectively; P < .001) and increased median duration of mechanical ventilation in survivors (5 days; interquartile [IQR], 2-11; 7 days; IQR, 4-14; and 9 days; IQR, 5-17, respectively; P < .001).
Etiology: Three clinical settings account for 75% of ARDS cases: 1. Sepsis syndrome – most important cause 2. Severe multiple trauma 3. Aspiration of saliva/gastric contents and it could also be a complication of pneumonia if left untreated known as aspiration pneumonia.
Other causes include shock, near-drowning, multiple transfusions and inhalation of irritants or toxic fumes that damage the alveolar epithelium.
Pulmonary Insults –> Aspiration of gastric contents, Embolism (thrombus, fat, or amniotic fluid), Miliary TB (rare), Diffuse pneumonia, Pneumocystis, Near-drowning, Toxic gas inhalation, Nitrogen dioxide, Chlorine, Sulfur dioxide, Ammonia, Smoke inhalation, Oxygen toxicity, Lung contusions, Radiation, High altitude, Hanging, Reexpansion, pneumonia (Mycoplasma, Legionella, fungi). Ten children who were accidentally exposed to excess chlorine (water turned yellow) at a swimming pool experienced substantial lung function impairment that was still apparent to some degree several months later (Am J Respir Crit Care Med 2006;174:545-549).
Systemic Insults –> Trauma, Sepsis, Pancreatitis, Shock (hemorrhagic, septic), Multiple transfusions, DIC, Burns, Drugs (narcotics, ASA, Chlordiazepoxide, Phenylbutazone, Colchicine, Ethchlorvynol, HCTZ, Paraldehyde, Lidocaine), TTP, Cardiopulmonary bypass, Venous air embolism, Head injury, Paraquat.
S/s: progressive pulmonary edema with refractory hypoxemia, decr lung compliance, progressive respiratory failure. Distinguished from cardiogenic pulmonary edema by a normal or low pulmonary capillary wedge pressure. Bilateral infiltrates, hypoxemia.
CXR: diffuse or patchy bilateral infiltrates that are initially interstitial but rapidly become alveolar, they tend to spare the costophrenic angles. Air bronchograms occur in about 80% of cases. Upper lung zone venous engorgement (flow inversion) is distinctly uncommon. Pleural effusion is small or nonexistent.
Dx Criteria: ARDS is the presence of pulmonary edema in the absence of volume overload or depressed left ventricular function.
Four main criteria for ARDS:
1. Hypoxia
2. Chest X-Ray: Bilateral diffuse infiltrates of the lungs
3. No cardiovascular lesion
4. PaO2/FiO2 ratio <200 = PaO2 (arterial oxygen tension) to fraction of inspired oxygen.
Other: PCWP <18 mmHg (normal). Acute onset in pt with predisposing problem.
The Berlin Definition:
(JAMA 2012;307:2526)
• The most notable change from the 1994 American-European Consensus Conference (AECC) definition is elimination of acute lung injury (ALI) as a category and creation of three categories of ARDS based on severity of hypoxemia (as defined by PaO2/FIO2: mild, moderate, severe).
• Mild—200 mm Hg < PaO2 /FiO2 d 300 mm Hg with PEEP or CPAP e 5 cm H2O.
• Moderate—100 mm Hg < PaO2 /FiO2 d 200 mm Hg with PEEP e 5 cm H2O.
• Severe—PaO2 /FiO2 d 100 mm Hg with PEEP e 5 cm H2O.
Other changes include a specified time frame for acute onset (symptoms developing within 1 week of a known clinical insult), clarification of radiographic findings, and removal of the requirement for absence of left atrial hypertension. The new definition requires that respiratory failure not be explained fully by cardiac failure or volume overload.
Tx: identifying and tx underlying condition. See: Sepsis. Supportive therapy, maximize O2 delivery (aim for SaO2 >90%) with mechanical ventilation (volume assist control). Stress ulcer and DVT prophylaxis. Albumin and Furosemide (aka “Lasix sandwich”) improves fluid balance and hemodynamics in hypoproteinemic pt’s with acute lung injury (Crit Care Med 2002;30:2175-82).
Low tidal volumes (6 cc/kg)
• Low TV: use <6ml/kg (based on IBW) tidal volume (~400-500ml) (normally use 12ml/kg) to avoid over-stretching the lungs (“volutrauma”) which decreases shunting, hypoxemia and alveolar capillary damage. Let the pH and CO2 be secondary concerns (permissive hypercapnia), if pH <7.15 give bicarb. The lowest possible FIO2 to keep PaO2 >60 mm Hg or the SaO2 >90%. Can use non-invasive positive pressure ventilation (NIPPV) if not obtunded and not delirious.
• PEEP to keep Pa02 ~ 60 mmHg with FI02 ~ 60%. PEEP (10-15cm) usually improves the oxygenation and allows for decr FiO2 (aim for <60%). Keep plateau pressure <30mmHg (measure in 10sec, first give 1sec pause, then check pressure at end insp, if high, adjust I:E ration, incr sedation, change to pressure cycled ventilation, may need to add paralysis). High levels of PEEP (13 cm) are not better than low levels of PEEP (8 cm) for ARDS (NEJM 2004;351:327-336, 389-391), both groups had a TV @ 6 mL/kg of body weight and plateau-pressure goal @ 30 cm of water.
Corticosteroids: A moderate dose of methylprednisolone (1 mg/kg/d), started early and tapered over several weeks, is beneficial in ARDS (Chest 2007;131:954-63) (more likely to be breathing without assistance at day 7, 54% vs. 25% and mortality and length of stay in the ICU were significantly reduced). No change in mortality at 60 and 180 days with IV methylprednisolone (0.5 mg/kg q6 hrs x 14 days and then tapered during the next several weeks (NEJM 2006;354:1671-84)….gave higher mortality if started >2wks after onset of ARDS (myopathy). Consider givning if due to radiation pneumonitis or fat embolism or if not improving after 7 days. Loading dose of methylprednisolone of 1 mg/kg, followed by a continuous infusion of 1 mg/kg per day for 14 days, 05 mg/kg per day for days 15 through 21 and then were tapered off the drug completely over the next seven days. Methylprednisolone down-regulates systemic inflammation and significantly improves the sx’s of ARDS as it reduced the median duration of mechanical ventilation from 9.5 days (placebo) to 5 days and improved the lung injury score by one point in 69.8% vs 37.5% (placebo) (Critical Care Congress of the Society of Critical Care Medicine. January 10 2006).
Ventilator-Induced Injury:
• Oxygen toxicity: Keep FI02 < 60%.
• Barotrauma: Keep peak pressures < 45 cm. Keep plateau pressures < 30 cm.
• Volutrauma: Use tidal volumes 6 cc/kg. Studies in animals: Augmentation of the inflammatory cascade with lung overdistension and with cyclical opening and closing of collapsible units. Increased lung neutrophil accumulation. Increased inflammatory mediators. Surfactant dysfunction.
• Protective Ventilation: Lower tidal volumes allow us to minimize the contribution of mechanical ventilation to ongoing lung injury and decrease mortality by 22-25% (From 40% to 32%). The only intervention known to decrease mortality as of 2012.
Alveolar recruitment maneuver: high peep for a short time to open up the alveoli by giving a CPAP of 40, peep of 30-40 for 30-120 sec, then give PC with a rate of 10 and peep of 20. Pt needs to be sedated and paralyzed. Must repeat with each disconnect from the ventilator.
Prone ventilation: Prone positioning during mechanical ventilation may not improve survival duration in patients with ARDS according to the results of a multicenter, unblinded, randomized controlled trial (JAMA. 2009;302:1977-1984, 2030-2032)…….”Based on the findings from the trial by Taccone et al combined with data from previous published reports, prone ventilation should not be used routinely in all patients with ARDS…..However, for a patient at imminent risk of death from hypoxemia, it makes sense to try prone ventilation, because multiple studies have demonstrated that it can increase oxygenation.” Typically used q12hr, a thought that up to 50% get a sustained response in oxygenation as shifts the infiltrates. If introduced early in pt’s with larger shunts and compliant chest walls, the acute ARDS pt benefits from prone ventilation, as it can produce sustained improvement in oxygenation Or put good lung down with pt on side (Crit Care Med 2002;30:1446-52).
• A randomized study of prone positioning for pediatric pt’s with acute lung injury was stopped early due to lack of benefit (JAMA. 2005;294:229-237, 248-250)…the author states that prone positioning should be tried in any child who has profound hypoxemia due to ARDS, provided reversible causes have been ruled out.
• Right ventricular pressure overload can be controlled in pt’s with severe adult ARDS by means of prone positioning (Chest 2007;132:1440-1446)….a residual PEEP level of 6 cm H2O is necessary.
• Can try inverse-ratio ventilation using a prolonged inspiratory time. May need pressors to maintain preload and augment CO. Keep albumin at >2.5 mg/dL with nutrition. Albumin for volume resuscitation and volume expansion in the critically ill is associated with increased mortality (Cochrane Database Syst Rev 2002;CD001208).
• The mortality rate associated with ARDS exceeds 50%, usually due to non pulmonary multiple organ system failure, often with sepsis (up to 80% if G-). Tx with exogenous surfactant does not increase the survival of pt’s with ARDS (NEJM 2004;853-855,884-892).
• Volume- and pressure-limited ventilation strategies should be used in managing adult acute lung injury and ARDS pt’s (TV target of 6ml/kg and peak pressure measured 0.5sec after end-inspiratory pause of <30cmH2O) (JAMA. 2005;294:2889-96)….the benefit of increased levels of PEEP and recruitment maneuvers is uncertain.
High Frequency Oscillation (HFO): allows delivery of a higher mean airway pressure (improving oxygenation) without high peak pressures. No data to prove benefit. Set FIO1 to same. Mean airway pressure at 3-5cm/H20 higher than MAP on conventional vent. Frequency of 3-8 Hz, usually 5Hz, adjusted according to CO2 levels. Power (oscillatory pressure amplitude) is titrated according to shake or wiggle of pt’s body (should wiggle to mid-thigh). I-E initially 33%. Bias flow at 20-30 LPM.
• HFOV did not prevent in-hospital mortality or shorten length of stay in adults with ARDS (N Engl J Med 2013:Jan 22;e-pub ahead of print)…..Although some institutions might offer it as a “rescue therapy” for patients with severe ARDS, but even this use is hard to justify in light of these trials.
If get hypoxia:incr FIO2, MAP by 2-3cm (with 30min between increments), pressures as high as 35-40 are used. Try recruitment maneuver (incr MAP to 40 cm H2O for 40 sec.
If get hypercapnia: increase power (to optimize wiggle), decr frequency (as low as 3 Hz). Wean / convert to conventional ventilation when FIO2 <50 and MAP 22 cmH2O.
The “lung open ventilation” (LOV) strategy seemed to improve oxygenation in acute lung injury (ALI) and ARDS, with fewer hypoxemia-related deaths and a decreased use of rescue therapies by the treating clinicians according to RCT with 983 pt’s (JAMA. 2008;299:637-645, 691-693)….the LOV strategy combined low tidal volumes, recruitment maneuvers, and high levels of PEEP vs an established low-tidal-volume strategy on mortality in patients with moderate and severe ALI.
Positive end-expiratory pressure (PEEP) therapy designed to increase alveolar recruitment while limiting hyperinflation did not significantly lower the rate of death for patients receiving mechanical ventilation but did improve lung function and reduce the duration of organ failure, according to a multicenter RCT (JAMA. 2008;299:646-655, 693-695). For finding the optimal PEEP for pt’s with ARDS, the use of esophageal pressure to guide PEEP has mixed results (NEJM 2008;359:2095).
Extracorporeal membrane oxygenation (ECMO): provides gas exchange while bypassing the lungs. In a multicenter U.K. study, researchers randomized 180 unconscious, intubated, and ventilated patients (age range, 18–65) with severe ARDS (Murray score >3.0 or pH <7.20) to referral to a single ECMO-capable center or to receive continued standard ventilator management found that 63% of patients referred for ECMO survived to 6 months without severe disability versus 47% of those allocated to conventional treatment (relative risk, 0.69)(Lancet 2009;Sep 16;e-pub ahead of print)……Patients who required high-pressure (>30 cm H2O) or high FiO2 (>80%) ventilation for more than 7 days and those with contraindications to limited heparinization (including intracranial bleed) were excluded.
Other: The lung collapse and hypoxemia that accompanies early ARDS might be reversed by bedside maneuvers such as stepwise increases in inspiratory airway pressures (Am J Respir Crit Care Med 2006;174:268-278).
• Transfusions may increase mortality in pt’s with ALI/ARDS (Chest 2007;132:1105)(adjusted odds ratio for death was 1.06 per unit of blood transfused)….clinicians should avoid transfusing hemodynamically stable pt’s with acute lung injury until the Hb level is in the range of 7 g/dL.
• Clinical and experimental findings indicate that the beta-agonist salbutamol (albuterol given IV) may be of value in treatment of patients with ARDS (Thorax 2008;63:189-190,215-220).
• Macrolide antibiotics appear to reduce mortality in patients with acute lung injury according to a post hoc analysis of data from an Acute Respiratory Distress Syndrome Network trial (Chest 2011;online November 23)…..erythromycin or azithromycin — starting within 24 hours and continuing for a median of four days…..Mortality was 23% with macrolide therapy compared to 36% without it…..lower 180-day mortality (hazard ratio 0.46; p=0.028) and faster discontinuation of mechanical ventilation (hazard ratio 1.93; p=0.009)….Fluoroquinolone, used in 90 patients, and cephalosporins, used in 93 cases, were not associated with improved outcomes, according to the authors.
**Ref: (Tx of ARDS. Chest 2001;120:1347-67) (Arch IM 1996;156:29) (Dis Mon 1996 May;42:270) (Lung 1995;173:139) (NEJM 1995;332:27)
Empyema / Parapneumonic Effusion (PPE):
Links: Info, Organsims, Stages, Dx | Types (Class), W/u & Tx | Pleurodesis | Anaerobic/ Aspiration |
Often defined as culture or gram-stain evidence of pleural-fluid microorganisms, or frank pus in the pleural space with pleural-fluid pH <7.2. Suspect empyema if fever persists despite appropriate antibiotic treatment of pneumonia. A  population-based study showed that pleural empyema complicated CAP in 0.7% of pt’s but did not affect these pt’s’ risk for in-hospital mortality (Am J Med 2006;119:877-83) (Streptococcus milleri among younger pt’s who are at risk for aspiration of oral contents — i.e., illicit drug users). Pleural infections differ bacteriologically from pneumonia and require different tx (Am J Respir Crit Care Med 2006;174:817-823)….50% of the community-acquired infections were streptococcal and 20% included anaerobic bacteria…..60% of hospital-acquired infections included bacteria frequently resistant to Abx’s with MRSA in 25% and Enterobacteriaceae in 18%. Same predisposition as Anaerobic/ Aspiration. Usually have cavitation of bacterial pneumonia distal to an obstruction. May be from hematogenous seeding of the lungs. 1/3 with pneumonia have an effusion, only 5% of effusions represent an empyema. Any pt with a parapneumonic pleural effusion >10mm in height on lateral decubitus CXR should undergo thoracentesis. Whenever a pt with a pneumonia is initially evaluated, one should ask if the pt has a parapneumonic effusion. This possibility should be evaluated with decubitus radiographs or U/S if the diaphragms are not visible throughout their entire length on the lateral radiographs or if it appears there is loculated pleural fluid. If pleural fluid is present and its thickness between the inside of the chest wall and the outside of the lung is more than 10 mm, the fluid should be analyzed within a short period. The definitive tx should be performed within the first 10 days of hospitalization.
population-based study showed that pleural empyema complicated CAP in 0.7% of pt’s but did not affect these pt’s’ risk for in-hospital mortality (Am J Med 2006;119:877-83) (Streptococcus milleri among younger pt’s who are at risk for aspiration of oral contents — i.e., illicit drug users). Pleural infections differ bacteriologically from pneumonia and require different tx (Am J Respir Crit Care Med 2006;174:817-823)….50% of the community-acquired infections were streptococcal and 20% included anaerobic bacteria…..60% of hospital-acquired infections included bacteria frequently resistant to Abx’s with MRSA in 25% and Enterobacteriaceae in 18%. Same predisposition as Anaerobic/ Aspiration. Usually have cavitation of bacterial pneumonia distal to an obstruction. May be from hematogenous seeding of the lungs. 1/3 with pneumonia have an effusion, only 5% of effusions represent an empyema. Any pt with a parapneumonic pleural effusion >10mm in height on lateral decubitus CXR should undergo thoracentesis. Whenever a pt with a pneumonia is initially evaluated, one should ask if the pt has a parapneumonic effusion. This possibility should be evaluated with decubitus radiographs or U/S if the diaphragms are not visible throughout their entire length on the lateral radiographs or if it appears there is loculated pleural fluid. If pleural fluid is present and its thickness between the inside of the chest wall and the outside of the lung is more than 10 mm, the fluid should be analyzed within a short period. The definitive tx should be performed within the first 10 days of hospitalization.
Organisms: most common with Streptococcus pneumoniae, Staphylodcoccus aureus, S. pyogenes, and mouth anaerobes.
3 stages: initial (exudative) phase the fluid is free flowing, the second (fibrinopurulent) phase get an accumulation of inflammatory cells and debri. Third stage (organizing), fibroblasts grow into the thickened pleural fluid to form an inelastic membrane (pleural peel) that may need decortication.
X-ray shows a radiolucent cavity surrounded by infiltrate, possible air-fluid level.
Dx: A chest radiograph with lateral decubitus is sensitive; computed tomography scan is also helpful. If empyema is being considered, an ultrasound guided thoracentesis should be performed. When pH is less than 7.2, glucose is less than 40 mg/dL, and lactate dehydrogenase exceeds 1000 IU/L, empyema is strongly suggested.
Infections of the Pleural Space: (IDSA Guidelines. Clin Infect Dis. 2013;published online:July 10) The infectious causes of pleural effusions have shifted from the traditional pneumonia pathogens of S. pneumoniae and S. pyogenes to polymicrobial infections in which anaerobic bacteria play a major role.
• Any significant accumulation of fluid in the pleural space should be sampled by thoracentesis. Specimens should be hand carried immediately to the laboratory or placed into appropriate anaerobic transport media for transport. In some institutions, bedside inoculation into blood culture bottles has become an established practice. This is acceptable providing that the manufacturer’s guidelines are followed with respect to the volume inoculated and whether supplementation is required to enhance recovery of fastidious pathogens such as S. pneumoniae. If blood culture bottles are used, an additional sample should be sent to the microbiology laboratory for Gram stain and culture of nonbacterial pathogens when indicated.
• Fluid should be sent for cell count, pH, protein, glucose, and lactate dehydrogenase (LDH). These values assist with the determination of a transudative or exudative process and in the subsequent management of the syndrome. For example, the following parameters suggest the need for drainage: pH <7.28; glucose <40 mg/dL; LDH >1000 IU/L or the presence of polymorphonuclear leucocytes (PMNs).
• Most infections result in an exudate or PMNs (empyema) within the pleural cavity. When tuberculosis or a fungal pathogen is thought to be the likely cause, a pleural biopsy sent for culture and histopathology increases the diagnostic sensitivity. Always notify the laboratory of a suspicion of tuberculosis so that appropriate safety precautions can be employed. An elevated adenosine deaminase level in the pleural fluid (>70 IU/L) in a patient with appropriate risk factors for tuberculosis has been shown to have a high sensitivity in high prevalence regions.
• A level <40 IU/L excludes the diagnosis. This marker of lymphocyte differentiation should be used in conjunction with hematologic and chemical parameters and other diagnostic tests such as NAAT, culture, and histology of a pleural biopsy. The performance of this assay in developed countries has been shown to be quite variable and is related to multiple factors including the type of method used, the likelihood of tuberculosis, and “false positive” results in patients with other causes of lymphocytic pleural effusion such as rheumatoid disease, mesothelioma, and histoplasmosis.
Ddx: cavitation due to malignancy or pulmonary embolism, TB, fungal or a pleural bleb.
Class 1: Insignificant (small): (<10mm thick) no thoracentesis needed. Tx with Abx and observation.
Class 2: Parapneumonic effusion >10mm thick: Thoracentesis shows a glucose is >40 mg/dL, pH >7 and G-stain & Cx negative.
Tx with Abx alone.
Note: pH is the preferred test for assessing the intensity of the inflammatory reaction (<7.20), it must be placed on ice and measured by a blood gas analyzer (can use glucose <60 mg/dL as a surrogate marker). No pH testing needed if have thick, turbid aka “purulent” pleural fluid as this alone substantially increases the risk of a poor outcome.
Class 3: Borderline Complicated: pH 7-7.2 and/or LDH >1,000 IU/L and glucose >40 with negative G-stain & Cx. CXR: no loculations (freely layers on the lateral decubiti film. Note: CT is insensitive at detecting loculations).
Tx with Abx & repetition of thoracentesis as needed.
Class 4: Simple complicated: pH <7 and/or glucose <40 and/or +G-stain or Cx. CXR: Not loculated, non purulent.
Tx with tube thoracostomy: Link: Chest Tube | (either large-bore rigid placed at bedside or small-bore flexible placed via fluoroscopy) & Abx or serial thoracentesis.
Class 5: Complex complicated pleural effusion: pH <7 or glucose <40 and/or +G-stain or Cx. CXR: Multiloculated, non purulent.
Tx with tube thoracostomy & thrombolytics (fibrinolytics such as 250,00 IU of Streptokinase qdX3 or 100,000 IU Urokinase qd X3-6d or tPA). In rare instances need VATS. The cost of 1 vial of streptokinase containing 250,000 IU is $127, and the cost of 1 vial of urokinase that contains 250,000 IU is $490.
Class 6: Simple empyema: frank pus. CXR: single loculation or freely flowing. Tx with tube thoracostomy with or w/o decortication.
Class 7: Complex empyema: frank pus. CXR: multiple locules. Tx with tube thoracostomy & thrombolytics. Often need thoracoscopy or decortication via VATS. Streptokinase (250,000 IU intrapleural BID x3d) does not improve survival or postpone surgery in pt’s with fibrinopurulent empyema that cannot be managed by chest-tube drainage (NEJM 2005;352:865-874), this reaffirms the early use of video-assisted thoracoscopy (VATS).
Tx of Empyema: Must be treated as a lung abscess. Needs immediate drainage. Has a 50% mortality in the elderly and immunocompromised. Prevent continued aspiration, postural drainage, Abx tx X 4-6wks. Can use fibrinolytics such as streptokinase or urokinase (better SE profile) to facilitate drainage. Or a CT guided catheter should be placed for drainage, with or w/o VATS in order to facilitate resolution. If not responding rapidly –> Tx of complicated parapneumonic effusion (PPE): has a pH <7, glucose <40mg/dL and an LDH >1000 IU/L. Chest tube drainage along with intrapleural instillation of streptokinase (see above) may improve outcome and reduce the need for surgery (9% versus 45%) in pt’s with empyema (Am J Respir Crit Care Med 2004;170:49-53).
Clogged Chest Tube: As proximal as possible, inject 5mg of tPa mixed in 45ml NS. Chase it with 60ml sterile saline. Keep tube clamped as 2-3hr, turn side-side q20min until unclamp. Keep chest tube in place until output is <100ml/d. A RCT with 454 pt’s fount that routine streptokinase (250,000 IU in 30ml NS q12hr x 6 doses) did not reduce mortality, need for surgical drainage or hospital length of stay (NEJM 2005l352L865-74).
Child: Chest drainage with intrapleural urokinase is just as effective as VATS in treating pediatric empyema, and it is significantly less expensive (Am J Respir Crit Care Med 2006;174:110-111,221-227).
Video-assisted thoracoscopic surgery (VATS): thoracic surgeon only makes a 1-2cm incision and uses direct visualization to disrupt loculations with possible lung resection and postoperative tube thoracostomy. Can be converted to a thoracotomy with decortication to strip thick visceral pleural rind. The first procedure performed to treat advanced empyema (ATS stage of IIA or higher) patients has a key impact on outcomes, as patients fare better when initial therapy is thoracotomy or video-assisted thoracic surgery (VATS) rather than simple drainage (Ann Thorac Surg 2009;87:1525-1531)……The treatment success rates with the two surgical operations, thoracotomy and VATS, were 89% and 81%, respectively. By contrast, pigtail drainage and tube thoracostomy yielded success rates of just 40% and 38%, respectively.
Thoracoscopy with Lysis of Adhesions: an option for the pt with an incompletely drained parapneumonic effusion. A chest CT scan should be obtained to provide anatomic information about the size and extent of the empyema cavity. The loculations in the pleural space can be disrupted, the pleural space can be completely drained, and the chest tube can be optimally placed. The pleural surfaces can be inspected to determine the necessity for further intervention such as decortication. If the pt is found to have a very thick pleural peel with a large amount of debris and entrapment of the lung, the thoracoscopy incision can be enlarged to allow for decortication if the procedure cannot be accomplished via thoracoscopy.
Decortication: involves the removal of all fibrous tissue from the visceral pleura and parietal pleura and the evacuation of all pus and debris from the pleural space. Decortication eliminates the pleural sepsis and allows the underlying lung to expand. Decortication is a major thoracic operation usually requiring a full thoracotomy incision ( median postoperative stay of 7 days) and should, therefore, not be performed on pt’s who are markedly debilitated.
Chronic drainage Tx: several open drainage procedures can be done. With the simplest procedure, segments of 1 to 3 ribs overlying the lower part of the empyema cavity are removed and 1 or more short, large-bore tubes are inserted into the empyema cavity. The tubes are subsequently irrigated daily with a mild antiseptic solution. The drainage from the tubes can be collected in a colostomy bag placed over the tubes. Alternatively, the empyema cavity can be packed with gauze. This procedure allows the pt to be freed from his attachment to the suction system and provides more complete drainage. A similar but more complicated procedure lines the tract between the pleural space and the surface of the chest with a skin and muscle flap after 2 or more overlying ribs are resected. The advantage of this open-flap (Eloesser flap) is that it creates a skin-lined fistula that provides drainage without tubes. It can therefore be more easily managed by the pt at home and permits gradual obliteration of the empyema space (median time for healing the drainage site is ~142 days).
Pleurodesis: typically used to prevent rapid accumulation of pleural fluid. Often use a talc slurry. Doxycycline is the can be used at an intrapleural dose of 1,500 mg. Can also use Bleomycin 60 U to sclerose the pleural space to obliterate it. Often add Lidocaine 1% ~250mg just prior to the sclerosing agent to alleviate pain. Can also send some pt’s out with indwelling catheters to periodically drain at home or a pleuroperitoneal shunt.
Chemical Pleural Sclerosis:
A bedside procedure used to obliterate the pleural space by inducing inflammation. A variety of substances have been used for pleural sclerosis. Currently the two most popular are talc and doxycycline. Talc pleural sclerosis has been shown to result in a lower recurrence rate of spontaneous PTX than doxycycline. Also, talc is generally less painful to the pt than doxycycline.
Indications: Persistent pneumothorax (persistent air leak with the lung expanded). Recurrent malignant pleural effusions. Recurrent spontaneous pneumothoraces (relative). Primary spontaneous pneumothorax (controversial).
Contra: Allergies to tetracycline, doxycycline, or talc.
Equipment: Kelly clamp. Sterile prep solution. Gauze. Sterile normal saline. Doxycycline or talc. 60-ml syringes (two).
Step #1: position pt initially supine. Pt must have a chest tube and Pleur-evac in place. Anesthetize with 1% lidocaine (50 ml).
Technique-Doxycycline: Obtain doxycycline 500 mg in 50 ml normal saline and 40 ml of 1% lidocaine in two separate 60-ml syringes. Alternatively, the lidocaine and doxycycline may be admixed in a 1:1 mixture and instilled together. Clamp the chest tube with a Kelly clamp near the entrance site at the skin. Sterile prep the distal portion of the chest tube at the connection with the Pleur-evac tubing, and then disconnect the chest tube from the Pleur-evac. Attach the 60-ml syringe containing the lidocaine to the end of the chest tube. Remove the Kelly clamp, instill the lidocaine through the chest tube into the pleural cavity, and reclamp the chest tube. Rotate the pt from side to side every 2 minutes for 10–15 minutes to distribute the lidocaine for anesthesia. Fill the other 60-ml syringe with the Doxy solution. Attach the doxycycline syringe to the chest tube, remove the Kelly clamp, and instill the solution into the pleural cavity. Clamp the chest tube. Repeat Steps until 300–400 ml of doxycycline have been administered. Rotate the pt to each of the following four positions every 30 minutes: Left lateral decubitus, Right lateral decubitus, Trendelenburg and Reverse Trendelenburg. After 4 hours, replace the chest tube back to Pleur-evac suction for at least the next 24 hours, until the lung has fully expanded and no air leak is present.
Technique—Talc: Obtain talc 5 g suspended in 250 ml normal saline and 40 ml of 1% lidocaine in two separate 60-ml syringes. Clamp the chest tube with a Kelly clamp near the entrance site at the skin. Sterile prep the distal portion of the chest tube at the connection with the Pleur-evac tubing, and then disconnect the pt’s chest tube from the Pleur-evac. Attach the 60-ml syringe containing the Lido to the end of the chest tube. Remove the Kelly clamp, instill the lidocaine through the chest tube into the pleural cavity, and reclamp the chest tube. Rotate the pt from side to side every 2 minutes for 10–15 minutes to distribute the lidocaine for anesthesia. Fill the other 60-ml syringe with the talc solution. Attach the talc syringe to the chest tube, remove the Kelly clamp, and instill the solution into the pleural cavity. Clamp the chest tube. Repeat Steps until all 250 ml of talc have been administered. Rotate the pt to each of the following four positions every 30 minutes: Left lateral decubitus, Right lateral decubitus, Trendelenburg and Reverse Trendelenburg. After 4 hours, replace the chest tube back to Pleur-evac suction for at least the next 24 hours, until the lung has fully expanded and no air leak is present. Nerve fibers in the pleural adhesions formed after talc pleurodesis may be be a source of chronic pain (Chest 2006;130:702-709).
Info: Pleurodesis with FDA-approved talc may lead to lung injury in a small but significant number of patients (Chest 2010;Jan)…they reviewed 142 thoracoscopic talc insufflations performed in 138 patients at the Lahey Clinic Medical Center in Burlington, Massachusetts…..All patients were treated with the talc preparation Sclerosol (has a higher proportion of particles smaller than 5 microns compared to Steritalc from Europe) in a median dose of 6 g (range, 2 to 8)……Dyspnea with increased oxygen requirements developed within 72 hours after 12 insufflations (8.5%)…..Eight patients also had new lung infiltrates on chest x-rays……”Based on the currently available evidence, we recommend the preferential use of ‘calibrated’ or ‘graded’ large particle talc, i.e., talc preparations from which the small particles have been mostly removed.” Dr. Gonzalez said.
ICD-9 Codes:
510.0 Empyema With fistula
510.9 Empyema Without mention of fistula
513.0 Abscess of lung
**Ref: (Pleural effusion. NEJM 2002;346:25) (Postgrad Med 1999; 105:7) (Medical and surgical tx of parapneumonic effusions. Chest 2000;18:1158-71) (Chest 1994;106:4) (Am Rev Resp Dis 1993;148:813) (Chylothorax, Chest 1993;102:586) (Useful tests on the pleural fluid in the management of pt’s with pleural effusions. Curr Opin Pulm Med. 1999;5:245-9) (Pleuritis and pleural effusions. Curr Opin Pulm Med. 1995;1:318-23) (Drugs and the pleura. Chest. 1999;116:212-21) (Pleural effusions in cardiovascular disease. Postgrad Med 2000;107:4) (Pleural Diseases, 3rd ed, W&W 1995;142) (Update: Management of Parapneumonic Effusions. Curr Opin Pulm 2003;10:336-342)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








