KEY POINTS
Risk of infection increases as the circulating absolute neutrophil count (ANC) declines below 1.0 and 0.5 × 109/L. The greatest risk of bacteremic infection occurs when the ANC is <0.1 × 109/L.
Cytotoxic therapy for remission-induction therapy for acute myeloid leukemia or conditioning therapy for bone marrow transplantation (high-risk patients) is associated with periods when the ANC is <0.1 × 109/L for 14 to 21 days. The time to marrow recovery (ANC >0.5 × 109/L) can vary from 21 to 42 days.
Intermittent administration of cytotoxic therapy for solid tissue malignancies or lymphoreticular malignancies (low-risk patients) is often associated with a neutrophil nadir at 10 to 14 days from beginning treatment and with periods of neutropenia (ANC <0.5 × 109/L) of less than 5 to 7 days. This pattern of neutrophil recovery influences the natural history of febrile neutropenic episodes.
Febrile episodes during neutropenia are defined by an oral temperature of ≥38.3°C (100°F) in the absence of other noninfectious causes of fever such as administration of blood products or pyrogenic drugs (eg, cytotoxic therapy, amphotericin B), the underlying disease, thromboembolic or thrombophlebitic events, or hemorrhagic events.
A single neutropenic episode may be characterized by one or more febrile episodes, of which one or more may represent infections.
Body sites most often associated with infection in the neutropenic patient are those associated with integumental surfaces (skin, upper and lower respiratory tract, and upper and lower gastrointestinal tract).
Antibacterial prophylaxis with oral fluoroquinolone agents such as ciprofloxacin or levofloxacin can reduce the frequency of febrile episodes and bacteremic events in patients with protracted neutropenia.
Patients undergoing remission induction for acute myeloid leukemia or bone marrow transplantation with a history of herpetic stomatitis or who are IgG seropositive for herpes simplex virus (HSV) are at risk for severe herpetic mucositis. Such patients should be considered for oral nucleoside analogue-based antiviral prophylaxis.
The recommended initial empirical antibacterial therapy for suspected infection in the febrile neutropenic patient is a broad-spectrum antibacterial regimen of an antipseudomonal penicillin or carbapenem administered as a single agent (monotherapy). Additional initial antibacterial agents such as aminoglycosides, fluoroquinolones, or vancomycin may be indicated for the initial management of severe sepsis/septic shock, pneumonia, or where antimicrobial resistance is suspected.
The median time to defervescence for febrile neutropenic patients at low- and high-risk for medical complications is 3 and 5 days, respectively.
INTRODUCTION
Critical care physicians are often called on to provide support for patients with various inherited or acquired defects in host defense that render them susceptible to potentially lethal infections. Patients with single host defense system defects (eg, congenital agammaglobulinemia) are susceptible to encapsulated respiratory pathogens such as Streptococcus pneumoniae that require opsonizing antibodies for clearance. In contrast, cancer patients undergoing potentially curative high-intensity myeloablative cytotoxic therapy acquire defects in multiple host defense systems that lead to increased susceptibility to different groups of pathogens normally contained and controlled by the absent or damaged systems.
The host immune response is mediated by the innate and adaptive defense systems. The former is mediated by pattern recognition receptors each with broad spectrum of specificities for genetically conserved and stable antigenic characteristics of pathogenic microorganisms.1 The latter, the adaptive immune system, is mediated by a diverse array of antigen receptors with random but narrow-spectrum specificities clonally distributed on B and T lymphocytes.2 Four broad categories of defects in host defense are clinically relevant: disruption of the integumental surfaces, quantitative neutrophilic phagocyte defects, diminished B-lymphocyte (humoral) function, and diminished T-lymphocyte system function. A working knowledge of the sources of failure in these host defense systems is particularly important for predicting the pathogens likely to be driving life-threatening infections. This, in turn, provides a basis for a rational approach to the choice of antimicrobial therapy. This chapter reviews the approach to managing suspected or proven infection in patients with multiple defects in host defense systems, with a particular emphasis on patients undergoing active myelosuppressive cytotoxic therapy, since this represents the largest group of immunocompromised patients who will require critical care services. Infections in patients with the acquired immunodeficiency syndrome (AIDS) are discussed in Chap. 69, and infections in those with organ or bone marrow transplantation are discussed in Chaps. 94 and 115; the problem of lung infiltrates in immunocompromised patients is covered in Chaps. 65 and 69.
Hematologists and oncologists have long recognized the existence of the direct relationship between dose and response in cancer therapy. Over the last 10 to 15 years, the supportive care strategies for cancer patients undergoing remission-induction or salvage therapy have improved sufficiently to permit the extension of dosing to the very limits of toxicity and beyond. For many malignant diseases, this has translated into significantly higher response rates and disease-free survival. Cure is now a goal that can be adopted realistically for many more patients with these diseases.
CANCER PATIENTS IN THE ICU
A greater number of cancer patients are being considered for admission to ICU for the management of critical illnesses developing as a function of the underlying caner or of its treatment.3 Combined modalities of anticancer treatment including aggressive surgical diagnostic and tumor debulking procedures, and targeted radiotherapeutic and systemic therapies have resulted in significant improvements in overall survival.4,5 During the years 1984 to 2000, hospital mortality rates for cancer patients admitted to ICU for mechanical ventilation were 70% to 85% and even higher, 95%, for hematopoietic stem cell transplant (HSCT) recipients requiring mechanical ventilation.6,7 Accordingly, cancer patients with critical illnesses have been at high risk for refusal for admission to an ICU setting.8
Recent experience has been more encouraging, however. Investigators began reporting reductions in the hospital mortality rates among cancer patients admitted to an ICU from 25-50% early this decade.7,9 Improved outcomes may be, in part, attributable to improved medical technologies such as noninvasive mechanical ventilation in the ICU and to better anticancer treatment, but also upon a better understanding of relevant prognostic factors contributing to outcome. The most important variable affecting prognosis and outcome for cancer patients is the status of the underlying malignancy at the time of ICU admission.10,11 Critical illness developing in patients with poor premorbid performance status and chronic end-organ damage in the setting of metastatic cancer represent a composite with the poorest overall outcome.8,12
Cancer patients have represented 9% to 15% of all patients admitted to general ICUs in Europe.13,14 Of these, solid tissue malignancies have constituted the majority (85%) and hematological malignancies comprised the remainder.14 Patients with hematological malignancies are more often admitted to the ICU with sepsis, whereas patients with solid tissue malignancies are more often admitted after surgery. Hematological malignancy patients are more severely ill than their solid tumor counterparts or those without cancer as measured by admission SOFA and SAPS II scores.14 Neutropenia upon ICU admission does not, in of itself, appear to affect outcome unless there is no myeloid reconstitution.15
Patients with newly diagnosed cancer may develop critical illness due to infection or cancer-related end-organ damage that requires ICU support prior to antineoplastic therapy. Invasive bacterial or fungal infections often occur in the setting of cancer-related myelosuppression with severe neutropenia due to myelophthisic processes, and opportunistic infections due to intracellular pathogens occur as consequence of disease-related immunosuppression with severe lymphopenia or functional hypogammaglobulinemia. Cancer-driven end-organ damage may include leukemic pulmonary leukostasis, intracranial lesions with mass effect, spontaneous acute tumor lysis syndrome, disseminated intravascular coagulation, hemophagocytosis syndrome, superior vena cava syndrome, malignant pleural or pericardial effusions, or bulky tumor masses with erosive effects upon vital structures.
In order to gain control of these progressive malignant processes, prompt administration of cytotoxic therapy in the ICU setting may be necessary. Under such circumstances, the 30-day all-cause mortality has been associated with requirement for vasopressors, mechanical ventilation, and hepatic failure.16 As for noncancer patients, the 30-day all-cause mortality increases with the number failing organs.
The 30- and 180-day all-cause mortality rates for cancer patients receiving primary cytotoxic therapy in the ICU have been reported to be of the order of 40% and 60%, respectively.16,17 The 30-day mortality rates are lower among patients with solid tumors compared to those with hematological malignancies but similar to those ICU patients without a cancer diagnosis.14 Overall, these observations have demonstrated that the administration of primary antineoplastic therapy in the ICU to critically ill cancer patients is feasible and may be associated with significant chances of survival. In contrast, administration of cytotoxic therapy in the ICU setting as salvage therapy to patients with relapsed cancer has been associated with prolonged survival in less than 10% of cases.17 Accordingly, the benefit of ICU-based cytotoxic therapy may be restricted to those at first presentation of cancer.
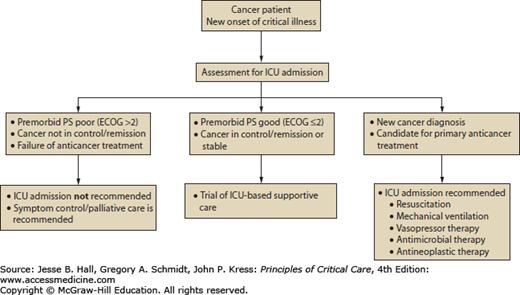
Three broad categories of admission criteria to ICU for cancer patients have been offered: postoperative care, management of medical emergencies related to cancer or its treatment, and monitoring during intensive anticancer treatments.10 The most common circumstances in which cancer patients may require access to ICU services include (1) respiratory failure, (2) postanesthetic recovery, (3) infection and sepsis, (4) bleeding, and (5) oncologic emergencies.18 Groeger and Aurora described three principles upon which decisions about deployment of ICU services for cancer patients occur. First, the intensive care clinician, in consultation with the referring cancer specialist and the patient, must try to balance the likelihood of survival from the critical illness against survival from the underlying malignancy. Second, the intensivist must understand whether the patient’s autonomy and expressed wishes are being respected as would be articulated in an advance care plan. Third, in the circumstances of limited resources the principle of distributive justice should be considered.10 As a framework to aid in the discussion of goals of care for cancer patients, including those suffering from a critical illness for which ICU services may be a consideration, Haines and colleagues classified patients in five categories: (1) those with newly diagnosed cancers, (2) those with a cancer diagnosis with the potential for cure, (3) those with controlled but incurable malignancy, (4) those who have failed specific treatment designed for cure or control, and (5) those being managed with palliative intent for symptom control.19 Based on this classification, types 1 and 2 cancer patients almost always would be candidates for ICU services, types 3 and 4 may be evaluated for such services on a case-by-case basis, and type 5 patients would not be candidates.18 An algorithm guiding decision making is offered for consideration in Figure 68-1.
DEFICITS IN HOST DEFENSES RELATED TO CANCER CHEMOTHERAPY
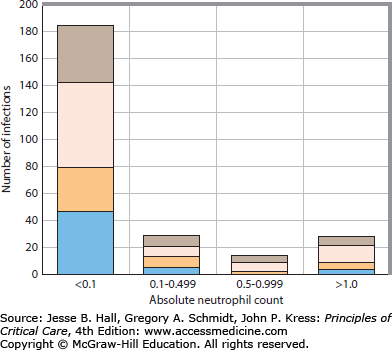
The absolute number of circulating segmented neutrophils (ANC) represents the most important single parameter predictive of the risk for life-threatening pyogenic infection.20 An ANC of 1.5 to 8.0 × 109/L can be considered normal for adults. As the ANC declines below 1.0 and 0.5 × 109/L, the risk of infection increases, with greatest risk for bacteremic infection at neutrophil counts below 0.1 × 109/L. For consistency, the terms severe and profound neutropenia refer to ANCs below 0.5 × 109/L and 0.1 × 109/L, respectively.21Figure 68-2 illustrates the relationship between the neutrophil count and infection for patients undergoing remission-induction therapy for acute leukemia.
FIGURE 68-2
The relationship between the ANC and occurrence of infection in 98 patients undergoing remission-induction therapy for AML. The proportions of the infections classified as possible infection, clinical infections, nonbacteremic microbiologically documented infection, and bacteremic infection are shown. The greatest risk for infection occurs when the ANC is <0.1 × 109/L. Possible infections, brown bars; clinical infections, pink bars; nonbacteremic microbiologically documented infections, beige bars; bacteremia, blue bars.
The ANC is calculated by multiplying the proportion of white blood cells (WBCs) that are segmented neutrophils on a Romanovsky-stained blood smear by the total number of WBCs in a specified volume of blood measured in an automated blood cell counter. Since neutropenic patients with acute leukemia undergoing cytotoxic therapy frequently have total WBC counts of <0.5 × 109/L, neutrophils may be difficult to detect on a manually reviewed stained smear; accordingly, the range of error for the procedure increases dramatically. Further, automated blood cell counters may give misleading results when abnormal cells such as leukemic blasts of similar size as segmented neutrophils are present in the circulation. This should dissuade the clinician from relying too heavily on a single ANC to judge the risk of infection. Rather, the clinical relevance of the ANC lies in the recognition of the range associated with a specific infection risk.
The pattern of change of the ANC has also a significant independent influence on infection risk. In an early study, 29% of the bacteremic episodes occurred as the neutrophil count was falling but before the ANC fell below 0.5 × 109/L.22 Therefore, with a falling neutrophil count, multiple observations over time are necessary to establish a pattern for the neutrophil profile and to estimate the relative infection risk. Survival of an infection during severe neutropenia is also intimately linked to marrow recovery and recovery of the circulating neutrophil count.23,24 The poorest outcomes for infectious episodes are observed among patients in whom the ANC continues to decline or fails to recover.25,26
The duration of severe neutropenia (ANC <0.5 × 109/L) is also related directly to infection risk. For example, bacteremic infections occur 3.5 and 5.4 times more often when neutropenia lasts 6 to 15 days and >15 days, respectively.27 The duration of neutropenia is related to the degree of hematopoietic stem cell damage caused by the underlying disease process and by myelosuppressive cytotoxic regimens. Following stem cell suppression, the peripheral neutrophil count falls at a rate directly proportional to the size of the circulating and marginated peripheral neutrophil pools and the size of the marrow storage pool of mature segmented neutrophils. Marrow recovery follows the recruitment of committed stem cell precursors of granulocytic, monocytic, erythroid, and megakaryocytic cell lines from the resting pluripotential stem cell pool.
Patients receiving pulse doses of chemotherapy on an intermittent cyclical basis for solid tissue malignancies or lymphoreticular malignancies sustain only temporary damage to the hematopoietic stem cell pool. The expected circulating neutrophil nadir occurs generally between days 10 and 14. Although the neutrophil nadirs may be <0.5 × 109/L, the duration of severe neutropenia is rarely longer than 5 to 7 days (median 3-5 days).28 For example, a patient receiving cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) beginning on day 1 of a 21-day cycle of initial treatment for an intermediate- to high-grade non-Hodgkin lymphoma might develop a febrile episode on day 12 in association with an ANC of 0.1 × 109/L. The patient’s neutrophil count would be expected to have reached its nadir, and a rise in circulating neutrophils would be predicted to occur between days 15 and 21. The likelihood that this prediction is correct is increased if a relative monocytosis is observed on the differential WBC count. The recovery of peripheral blood monocytes precedes that of circulating neutrophils in chemotherapy-induced aplasia and often heralds the recovery of the ANC.
In general, the more dose-intensive myelosuppressive regimens are associated with more hematopoietic stem cell damage and longer durations of neutropenia. Standard remission-induction regimens for acute myeloid leukemia (AML) are composed of anthracycline drugs such as daunorubicin administered in intravenous doses of 30 to 90 mg/m2 daily over 3 days and an antimetabolite, cytarabine, administered as an intravenous bolus or as a continuous infusion at doses of 100 to 200 mg/m2 daily over 5 to 7 days (a “7 + 3” regimen).29 These regimens predictably produce periods of profound myelosuppression, in which a median of 24 to 26 days passes until the circulating neutrophil count rises above 0.5 × 109/L.30-32 If more than one cycle of therapy is required to achieve a complete remission, then the median time until marrow recovery may be prolonged by as long as 40 days (range 33-60 days). This additional period of myelosuppression is associated with a significant increase in infectious morbidity.30,33
Intensive regimens using high-dose cytarabine (HDARA-C) in doses 15 to 30 times that for standard induction regimens have been successful for salvage therapy of relapsed or resistant leukemia, for initial remission-induction therapy, and for post-remission consolidation therapy for acute leukemia.30 The median time until neutrophil recovery following administration of HDARA-C (3.0 g/m2 infused over 1 hour every 12 hours for 12 consecutive doses) is 28 days.30 Surprisingly, the overall period of myelosuppression is not substantially longer than for standard induction regimens. The time from day 1 of HDARA-C post-remission consolidation until the development of severe neutropenia is a median of 10 days (compared to a median of 4 days for primary induction with a “7 + 3” regimen).30 Accordingly, the overall duration of severe neutropenia for patients receiving post-remission consolidation with HDARA-C may be shorter than for a “7 + 3” primary induction by 4 to 6 days.30
Patients undergoing hematopoietic stem cell transplantation are conditioned for the stem cell infusion through the administration of cytotoxic therapy alone or in conjunction with irradiation in an attempt to reduce tumor burden and to suppress the host immune system in order to permit stem cell engraftment.34 The intensities of the commonly used conditioning regimens vary significantly and have differential impacts upon the degree of tumor reduction, immunosuppression, toxicities, and treatment-related mortality. In the case of acute leukemia, increased conditioning intensity may reduce leukemia recurrence, but at the expense of increased toxicity.35 In the case of allogeneic HSCT, a reduction in the intensity of the conditioning regimen not only reduces toxicities, it reduces the anticancer cytoreductive effect; the anticancer effect must be derived from the graft-versus-tumor effect. Conventional myeloablative conditioning regimens are based on cyclophosphamide (Cy) plus either busulfan (Bu) or total body irradiation (TBI), the so-called BuCy and CyTBI regimens. More recently, a reduced intensity approach based on fludarabine (Flu), an immunosuppressive antimetabolite, used with reduced doses of the alkylating agents Cy or Bu, or reduced doses of TBI has become very widely used among transplant centers. The term, myeloablative as applied to conditioning regimens in HSCT is defined by the administration of cytotoxic agents in doses sufficient to preclude spontaneous autologous hematopoietic recovery.36 In contrast, the term reduced intensity conditioning (RIC) is defined by the administration of cytotoxic agents in doses that produce prolonged but not irreversible myelosuppression and cytopenia; however, stem cell support is required in order to mitigate excess aplasia-related morbidity and mortality.36 In contrast to the classical myeloablative conditioning regimens, RIC conditioning regimens typically have dose reductions in the alkylating agent or the TBI of ≥30%. Examples of such regimens include Flu combined with melphelan,37 busulfan,38 or thiotepa,39 or reduced dose TBI.40 The term nonmyeloablative conditioning is applied to a regimen that will produce temporary hematopoietic suppression and minimal cytopenia without the need for stem cell support.36 Examples of such regimens include FluCy,41 TBI at a dose of 1 to 2 Gy,42,43 antithymocyte globulin, and total lymphoid irradiation (TLI).44 Basic understanding of the conditioning regimen can help predict the duration of myelosuppression36 or the need for mechanical ventilation.45
Patients receiving less myelosuppressive treatments have a lower risk for severe neutropenia and neutropenic fever. Between 85% and 95% of patients undergoing “7 + 3”-based AML treatments are expected to develop neutropenic fever; whereas, approximately 1%, 4%, 5% to 6%, 10%, 12%, 23% of patients receiving standard cytotoxic regimens for prostate, breast, colorectal, lung, ovarian, and germ cell cancers, respectively, over the course of several cycles of treatment.46 Similarly, between 15% and 26% of patients undergoing multiagent chemotherapy for Hodgkin and non-Hodgkin lymphoma may develop a neutropenic fever over the course of therapy; however, the majority of such episodes occur within the first 1 to 2 cycles.47 Independent risk factors for neutropenic fevers and for complications of neutropenic fevers (including prolonged hospitalization, need for critical care services, and death) have included advanced age (≥65 years), type of cancer, advanced cancer stage and large tumor burden, and increasing number of comorbid conditions (including hypertension, chronic airflow obstruction, pneumonia, previous invasive fungal infection, and sepsis).48,49
The incidence of neutropenic fever presenting to the emergency department (ED) is relatively uncommon.50 Even more uncommon is the requirement for ICU services in this context. Among 777,876 ED visits in 47 French hospitals over a 6-month period, only 198 (0.03%) satisfied the case definition (ANC <0.5 × 109/L and a core temperature >38.3°C) for neutropenic fever.51 Of these, patients with solid tumors accounted for 56% and hematological malignancies for 44%. Severe sepsis or septic shock was the presenting problem in the ED 89 of 198 patients (45%). A total of 18 patients, 9% of the total group of febrile neutropenic patients and 20% of the 89 presenting with severe sepsis or septic shock, were admitted to the ICU.51 These observations suggest that almost half (45%) of the cases of neutropenic fever present to the ED for care will have potentially life-threatening severe neutropenic sepsis of which one in five may require ICU admission.
The Multinational Association for Supportive Care in Cancer (MASCC) developed and validated a scoring system to discriminate febrile neutropenic patients at high or low risk for medical complications that would either require hospitalization or prolong an admission.52,53 In one report, 85% of those patients defined by this scoring system as high risk (score <21) also had medical complications sufficiently serious to warrant ICU admission.54 The MASCC score is a useful tool for the identification of those febrile neutropenic patients who may be at greater risk for complications that may require critical care services.
Hemodynamic instability during the evolution of the neutropenic fever is one of the common reasons for ICU admission. Effective antibacterial therapy within 1 hour of hypotension is associated with a survival advantage among patients with septic shock.55 Among patients with acute community-acquired pneumonia, initial antibacterial therapy administered early in the ED (door-to-needle time, mean 3.5 ± 1.4 hours) rather than on an inpatient unit (door-to-needle time, 9.5 ± 3.0 hours) was independently associated with a significantly shorter duration of hospitalization56 and a lower hospital-based all-cause mortality.57
A wide range of times from triage in the ED to antimicrobial administration have been reported (102-254 minutes).58-64 Current guidelines recommend that antibacterial therapy be initiated early (within 60-120 minutes) in neutropenic patients presenting with severe sepsis.46,51,65
The remission-induction regimens commonly used for acute leukemia have important immunosuppressive effects in addition to the myelosuppressive effects discussed above. Anthracyclines and similar agents (eg, doxorubicin, daunorubicin, idarubicin, epirubicin, amacrine, mitoxantrone, and rubidazone), antimetabolites (eg, cytarabine, methotrexate, thioguanine, and mercaptopurine), and alkylating agents (eg, cyclophosphamide, ifosfamide, melphalan, busulfan, and platinum analogues) have profound suppressive effects on the numbers of circulating T- and B-lymphoid cells that parallel the acquired functional defects in cell-mediated and humoral immune mechanisms. The consequences of these effects are reflected by an increased susceptibility to pathogens normally controlled by these mechanisms. The ultimate impact on immune responsiveness appears to depend on the schedule of administration.
T-Lymphocyte Function: Indications from in vitro testing of lymphoid cell responsiveness to mitogen-induced blastogenesis suggest that T-cell function may be moderately depressed in patients with acute leukemia. Among patients undergoing remission-induction therapy for acute leukemia, decreased cell-mediated immune responsiveness can be detected for up to 6 months following chemotherapy-induced remission.66,67 In some patients, immune function decline may herald a relapse.67
Patients who have received purine analogue therapy for chronic lymphocytic leukemia, specifically fludarabine, have prolonged qualitative and quantitative T-lymphocyte defects, and, in addition, B lymphocytopenia and monocytopenia. As result, there are enhanced susceptibilities to pyogenic bacteria (Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa), opportunistic bacteria (including Listeria monocytogenes, Nocardia spp, and Mycobacterium spp), invasive fungal pathogens (such as Candida spp, Aspergillus spp, Pneumocystis jirovecii, and Cryptococcus spp), and DNA virus infections (such as herpes group viruses including varicella-zoster virus, herpes simplex, and cytomegalovirus).68
The clinical consequences of T-cell dysfunction vary with the underlying disease and the cytotoxic regimen. For example, Pneumocystis jirovecii infection is an uncommon phenomenon among adult patients undergoing remission induction for AML but relatively common among children undergoing consolidation and maintenance-phase chemotherapy for acute lymphoblastic leukemia (ALL).69-71 An intermediate degree of risk for pneumocystosis appears to be present in those undergoing bone marrow allografting or autografting. The immunosuppressive potential of the conditioning regimens for HSCT appears to be greater than that associated with AML induction regimens. Accordingly, most centers managing these patients recommend administering primary prophylaxis for P jirovecii in HSCT recipients or patients with ALL. These infections rarely occur during the primary period of myelosuppressive therapy–induced neutropenia.
Monoclonal Antibodies There have been a number of anti-T-lymphocyte monoclonal antibody products that have been licensed by the Federal Drug Association (FDA) for the treatment of lymphoma, AML, breast cancer, colorectal cancers, rheumatoid arthritis, and Crohn disease. Treatment with some of these products has been associated with a higher risk for opportunistic infections due to CMV, Epstein-Barr virus, BK polyoma virus, and invasive fungal infections.
Tumor necrosis factor-α (TNFα) is a mediator that stimulates the release of pro-inflammatory cytokines (such as interleukin [IL]-1β, IL-6, and IL-8), monocyte chemoattractant protein-1, adhesion molecules, and phagocyte and T-lymphocyte activators.72 TNFα blockers such as infliximab, etanercept, and adalimumab have been used in the management of severe autoimmune disorders, chronic inflammatory bowel disease, steroid-refractory graft-versus-host disease, and solid organ transplant graft rejection. These products have been associated with a two-to-threefold increase in serious infectious complications, particularly in patients with rheumatoid arthritis. 73 Infectious complications have included mycobacterial diseases, due to both Mycobacterium tuberculosis74 and nontuberculous mycobacteria.75 The median time of onset of clinical infection from the initiation of treatment has been 12 weeks.74 Such infections have tended to be fulminant and involve extrapulmonary sites. Invasive fungal infections such as histoplasmosis, invasive candidiasis, invasive aspergillosis, coccidiodomycosis, and cryptococcosis have also been associated with TNFα blocking agents.76
Immune modifiers of T-lymphocyte function are often deployed in hematologic and solid organ transplantation to treat or prevent graft-versus-host disease or graft rejection, respectively. These products include antithymocyte globulin (ATG) prepared from rabbits (rATG) or horses (hATG), the non-T lymphocyte depleting anti-interleukin-2α receptor preparations (daclizumab and basiliximab), and alemtuzumab. All ATGs produce a dose-dependent depletion of T lymphocytes. Treatment may result in significant lymphopenia for up to a year. For example, there is a direct dose-dependent relationship between ATG administration and opportunistic CMV infection. Pyrexia and TNFα release after the administration of ATG stimulates cellular nuclear factor κB (NFκB) binding to the promoter region of the immediate early antigen gene of CMV.77,78 CMV infection after ATG administration depends on the ATG product used, the dose administered, the pretransplant donor and recipient serologic status, and use of CMV prophylaxis.79 The incidence of cancers, particularly posttransplant Epstein-Barr virus transformed lymphoreticular disorders (PTLD), is increased in association with ATG administration and with coinfection by CMV. The highest risk for PTLD is among EBV seronegative organ transplant recipients receiving a transplant from an EBV seropositive donor.79 Similarly, there is an increased dose-dependent risk for BK polyomavirus viremia, viruria, and nephropathy with ATG therapy.80,81 These processes may confound the assessment and management of critically ill transplant recipients unresponsive to broad-spectrum antibacterial therapies and should be considered in this context.
The monoclonal IL-2α-chain receptor (CD25) antagonists, basiliximab and daclizumab, inhibit lymphocyte activation, differentiation, and proliferation. Treatment results in a complete saturation of the receptor for 6 to 12 weeks. While little effect of these agents on bacterial, EBV, or fungal infection has been recognized, CMV shedding does appear to be somewhat increased in solid organ transplant recipients.79,82 Increased infection-related mortality has been observed among daclizumab recipients for steroid-refractory GvHD in allogeneic stem transplantation.
Alemtuzumab, a humanized monoclonal antibody preparation, targets cell surface CD52 present on the surface of normal and malignant T and B lymphocytes, monocytes, and NK lymphocytes. The product has been used in chronic lymphocytic leukemia and T-cell depletion of allogeneic stem cell products, resulting in a sustained reduction in both CD4 and CD8 T lymphocytes within 4 weeks of administration that may last as long as 9 months.83 The infectious complications described in association with the use of alemtuzumab include reactivation of herpesvirus infections (human cytomegalovirus, varicella-zoster virus, herpes simplex virus), new respiratory virus infections, and invasive fungal infections (including pulmonary pneumocystosis, invasive candidiasis, and invasive aspergillosis).68,82,84 Also, invasive zygomycoses, tuberculous and nontuberculous mycobacterial infections have been observed.82 In a retrospective analysis of post-engraftment infections in allogeneic stem cell transplant recipients conditioned with alemtuzumab or antithymocyte globulin, the incidence of non-CMV infections was significantly higher among alemtuzumab recipients.85 Moreover, the proportion of patients developing CMV disease or BK virus associated hemorrhagic cystitis were markedly higher among alemtuzumab recipients.85
B-Lymphocyte Function: Modern cytotoxic therapy for acute leukemia appears to have a more profound effect on humoral immune competence than on T-lymphocyte function. Serum immunoglobulin concentrations and the efficiency of new antigen-induced immunoglobulin synthesis have been observed to decline following institution of remission-induction therapy, reaching a nadir at approximately 5 weeks. It has been difficult to separate the effects of the underlying malignant disease from the effects of the cytotoxic therapy. There does not appear to be a prognostically useful parameter of T- or B-cell function that predicts infection risk in neutropenic patients analogous to the predictive value of the ANC for pyogenic bacterial or fungal infection. However, presence of hypogammaglobulinemia may help identify increased risk of infection by encapsulated bacteria.
Rituximab, a chimeric murine-human monoclonal IgG1 antibody preparation administered for treatment of B-cell non-Hodgkin lymphoma, targets CD20 on the surface of normal and malignant B lymphocytes, leading to a predictable depletion of these cells over a 6- to 9-month period. This agent does not affect CD3, CD4, CD8, or natural killer T-lymphocyte populations. Based on a systematic review of five randomized controlled trials on the treatment of non-HIV patients with non-Hodgkin lymphoma, no incremental risk for infections has been observed.86 This notwithstanding, two recently published systematic reviews with meta-analyses have suggested an enhanced risk for serious grade 3 to 4 infectious complications associated with the use of rituximab for maintenance therapy in non-Hodgkin lymphoma patients.87,88 Case reports and small series have reported some opportunistic infections associated with the use of rituximab, including tuberculosis, progressive multifocal leukoencephalopathy, babesiosis, pulmonary Pneumocystis jirovecii infection, enteroviral gastroenteritis, cytomegalovirus infection, reactivation of hepatitis B virus infection, disseminated varicella-zoster, and parvovirus B19–related pure red cell aplasia.
Integumental Barriers: Integumental barriers are among the most important and most often damaged defense systems for cancer patients. These barriers include the epithelial surfaces of the skin, the upper and lower respiratory tract, the upper and lower gastrointestinal (GI) tract, and the mucosal surfaces lining the genitourinary tract. In critically ill patients, the barrier function of these surfaces may also be compromised by procedures such as percutaneous intravenous catheterization, endotracheal intubation, endoscopic procedures, nasogastric intubation, and indwelling urinary catheterization (Table 68-1).
Integumental Defects
| Damage to mucosal surfaces |
| Endotracheal tube |
| Nasogastric tube |
| Cytotoxic therapy–induced damage to gastrointestinal and respiratory epithelial barriers |
| Endoscopic diagnostic procedures |
| Damage to skin and supporting structures |
| IV catheters |
| Peripheral IV lines |
| Indwelling central venous catheters |
| Indwelling urinary catheters |
| Biopsy sites |
| Bone marrow |
| Lymph nodes |
| Skin |
Integumental damage secondary to cytotoxic therapy has become more prevalent as the dose intensity of the remission-induction regimens has increased.89 The epithelial surfaces of the GI tract appear to be at greatest risk. The antiproliferative effect of therapy prevents cell recruitment into mucosal areas denuded by erosion or by cellular attrition, resulting in the appearance of superficial erosion and ulceration. The absorptive capacity of the GI mucosa may also be impaired significantly among recipients of regimens such as HDARA-C, and both anatomic mucosal disruption and absorptive dysfunction appear to temporally parallel that of the neutrophil profile.
A high proportion of patients receiving cytoreductive therapy also experience painful, often debilitating inflammatory lesions within the oral cavity.90 The tissues of the periodontium, gingival surfaces, oral mucosa, and mucosal surfaces of the upper and lower bowel are affected.89 Cytotoxic regimens affect the developing basal epithelial cells of the oral mucosa in a manner that parallels the effect on the marrow system cell pool and the intestinal mucosal surface.91 Mucosal atrophy, cytolysis, and denudation of the mucosal surface result in the painful foci of local ulceration typically observed 4 to 7 days after administration of cytotoxic agents, which usually resolve spontaneously between days 14 and 21.90,92
Cytotoxic therapy–induced intestinal mucosal damage has been described in three stages.91 The first stage of initial injury begins during the first week of cytotoxic therapy and is characterized by replacement of the normal crypts and mucus-secreting goblet cells by atypical undifferentiated cells. The second stage represents progressive mucosal injury that occurs during the second and third weeks. This stage is characterized histologically by cellular necrosis, lack of mitotic activity, and focal loss of villous surfaces and clinically by abdominal pain, diarrhea, electrolyte loss, and invasive infection. The third stage of cellular regeneration occurs after the third week and is characterized by resumption of mitotic activity and cellular proliferation in the crypts with subsequent repopulation of the denuded surfaces by differentiated cells.
The maximum cytotoxic therapy–induced intestinal epithelial damage occurs in the second week between days 10 and 14.93-95 This corresponds to the median time of onset of bacteremic infection on day 14 due to the microorganisms that normally colonize these surfaces.96 To a limited extent, the type of pathogens recovered in bacteremic infections can be predicted from the pool of microorganisms colonizing damaged mucosal surfaces. Oral mucosal ulceration, particularly that involving periodontal tissues, is often associated with viridans group streptococcal bacteremia.97,98 Colonic mucosal damage is more likely to be associated with aerobic gram-negative bacillary infection with Escherichia coli, Klebsiella species, Pseudomonas aeruginosa, or opportunistic yeasts when these pathogens are colonizing the lower GI tract.96
Mucositis not only predisposes patients to invasive infection, but also imposes a significant cost with respect to the resources needed to manage the consequences of mucositis.99,100 Recent cost estimates suggest that an episode of severe mucositis may cost an average of $7985 (Year 2002) per patient.100 Neutropenia with or without infection is estimated to cost an average of $9316 (Year 2002) per inpatient.
INFECTIONS AND BACTERIAL PATHOGENS CAUSING NEUTROPENIC FEVERS
A review of bloodstream infections occurring in patients with hematological malignancies over a 14-year period in a tertiary cancer center in Sweden noted that gram-negative bacilli accounted for 45% and gram-positive organisms accounted for 55%.101 Of note in this experience was the rising incidence of enterococcal bloodstream infections due to penicillin-resistant E faecium and the high 30-day mortality (24%) compared to other gram-positive 30-day mortality rates (∼15%).101
In an Irish 5-year experience in febrile neutropenic cancer patients, 20% of blood cultures revealed 172 isolates of which 123 (71%) were gram-positive organisms, 48 (28%) were gram-negative bacilli, and 2 were yeasts.102 Of the gram-positive organisms, 93 were Staphylococcus spp, 10 were Streptococcus spp, 11 were Enterococcus spp, and 9 were predominantly gram-positive bacilli. The staphylococci were coagulase negative in 65 and S aureus in 28, of which 25 (89%) were methicillin resistant. This highlights the high incidence of methicillin resistance in this population and has implications for the choice of initial empirical antibacterial therapy.
The infections documented among febrile neutropenic patients have been classified as microbiologically documented with the identification of a pathogen and a focus of infection; as clinically documented with the identification of a clinical focus of infection without isolation of a putative pathogen; and as an unexplained fever wherein neither a clinical focus nor a pathogen are identified.103 Among febrile neutropenic cancer patients not receiving fluoroquinolone chemoprophylaxis managed during the early 1990s, Cornelissen and colleagues reported that microbiologically documented infections were observed in 33% of patients with gram-negative infections comprising 18%, gram-positive infections in 9%, and mixed gram-negative and gram-positive in 6%.104 Forty-two percent of patients had clinically documented infections and the remaining 24% had unexplained fevers. Among a similar group of patients who had received ciprofloxacin chemoprophylaxis, there were no gram-negative infections. Gram-positive infections were observed in 38% of patients, clinically documented infections in 47% of patients, and unexplained fevers in only 15%. Fluoroquinolone antibacterial chemoprophylaxis can reduce the risk for invasive gram-negative infections in patients at high-risk for such infections in an environment where the prevalence of gram-negative resistance to fluoroquinolone antibacterial agents is low.21,105
A more recent study wherein the investigators identified all potential clinical foci of infections reported the GI tract as the focus of infection in 41% of patients with the oropharynx accounting for 70%, esophagus 3%, clinical neutropenic enterocolitis 17%, and perirectal soft tissue infection 10%.106 Other foci included the respiratory tract in 10%, urinary tract in 6%, and skin and soft tissue in 10%. Of the skin foci, indwelling central venous catheter sites accounted for 59%, folliculitis for 6%, and cellulitis for 35%.106 Unexplained fevers accounted for only 10% of cases and microbiologically documented bloodstream infections in 23% of patients of which gram-negative bacteremia accounted for 37%, coagulase-negative staphylococcemia 19%, streptococcemia 27%, and other gram-positive microorganisms in 16%.106 These observations illustrate that with clinical diligence, clinical foci of infection may be identified in the majority of febrile neutropenic patients receiving cytotoxic therapy.
APPROACH TO NEUTROPENIC FEVER
Fever is the hallmark of infection for most patients with prolonged severe neutropenia; the definition of fever due to suspected infection in a neutropenic patient has varied greatly.25-27,97,106-117 The International Antimicrobial Therapy Cooperative Group (IATCG) of the European Organization for Research and Treatment of Cancer (EORTC),27,112,113 the Intercontinental Antimicrobial Therapy Study Group,115 and others114 have used an oral temperature of >38°C (100.8°F) sustained over a 12-hour period or a single oral temperature of >38.5°C as the criterion for infection-related fever. The recently published German guidelines define an unexplained fever by a single oral temperature of ≥38.3°C or ≥38.0°C lasting over an hour or measured twice within 12 hours.118 The National Cancer Institute of Canada Clinical Trials Group also has used a single oral temperature of >39°C together with chills or rigors instead of a single temperature of >38.5°C.26,119 In order to avoid the administration of antimicrobial therapy for noninfectious causes of fever, a stipulation that other causes of noninfectious fever should be excluded (eg, blood products, pyrogenic drugs such as amphotericin B, thrombophlebitis, or hematoma) is often added to the definition. The Infectious Disease Society of America has suggested that a single oral temperature of ≥38.3°C (101°F) in the absence of other obvious environmental causes would be a reasonably safe working definition for an infection-related fever in neutropenic patients.65,120
The extent to which characteristics of the febrile episode predict a bacteremic event has been somewhat variable in different studies; however, most agree that initial oral temperatures of >39°C (102.2°F), shaking chills, shock, initial ANC of <0.1 × 109/L, and initial platelet count of <10 × 109/L are somewhat predictive of gram-negative bacteremia. Viscoli et al27 demonstrated an 8.4-fold increase in risk for bacteremic infection in neutropenic patients with initial temperatures of >39°C (102.2°F). The duration of fever prior to evaluation, however, does not appear to influence the risk of gram-negative bacteremia.22
The risk of developing a febrile neutropenic episode during each cycle of outpatient cancer chemotherapy for solid tissue malignancies or lymphoreticular malignancies is generally low. 121 However, this risk increases with the number of cycles administered122 and with the dose-intensity of the regimen selected.123 In a study of patients with lymphoma from the MD Anderson Cancer Center over 30 years ago, the cumulative incidence of febrile neutropenic episodes increased from 12% among recipients of cyclophosphamide, vincristine, and prednisone (COP) to 27% among patients receiving a regimen where doxorubicin (hydroxydaunorubicin) was substituted for the cyclophosphamide (HOP) and 46% among recipients of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).124 In recent studies, the incidence of infection among CHOP recipients has been lower, approximately 15% of all cycles.125 Similar to the study by Feld and Bodey,124 the addition of cytotoxic agents to a chemotherapy regimen has the effect of increasing the likelihood of myelosuppression. For example, the addition of paclitaxel or docetaxel to carboplatin for the treatment of gynecological malignancies increased the incidence of severe neutropenia (ANC <0.5 × 109/L) from <1% to 7% and 73%, respectively.126 The addition of irinotecan to carboplatin and paclitaxel increased the incidence of severe neutropenia from 7% to 61% but did not enhance the odds of developing a febrile neutropenic episode.126 The median incidence of febrile neutropenic episodes in patients with small cell lung cancer is reported as 35% for recipients of cyclophosphamide, doxorubicin, and etoposide (CAE) compared with 18% for recipients of a less myelosuppressive regimen containing cyclophosphamide, doxorubicin, and vincristine (CAV).127 In addition to neutropenia, the propensity of a given regimen to induce mucosal damage resulting in mucositis correlates directly with the incidence of febrile neutropenic episodes. The severity of mucositis is directly related to the incidence of febrile events, the duration of hospitalization, the costs of medical care, and treatment-related mortality.99
Febrile neutropenic episodes occur in 70% to 90% of patients receiving cytotoxic therapy for acute leukemia and bone marrow transplantation.119,128,129 This difference is due to the more prolonged cytotoxic therapy–induced myelosuppression and greater intestinal epithelial damage in these patients.93
Prolonged neutropenia may be punctuated by one or more febrile episodes, and a single febrile neutropenic episode may represent more than one infectious process. For example, febrile neutropenia associated with a viridans group streptococcal bacteremia may not defervesce promptly despite appropriate antibacterial therapy and documentation of a microbiologic cure on the basis of subsequent sterile blood cultures. This phenomenon of a persistent febrile state may occur in association with the concomitant administration of pyrogenic blood products, the presence of a coexisting infection such as a herpes simplex virus (HSV) mucositis, or a possible fungal superinfection. It is frequently impossible to distinguish clinically the boundaries defining separate sequential infectious processes by the pattern of fever unless a clear pattern of defervescence is seen between one infection and the next. This is particularly frustrating when managing febrile neutropenic patients without a clinical focus of infection or a defined pathogen.
The diagnosis of a febrile state in a neutropenic patient requires a complete but directed clinical history and physical examination designed to identify potentially infected foci for which those patients are at special risk.
Important historical facts may be obtained from the patient, significant others, and the medical record. The degree of neutropenia and the day of the chemotherapy cycle are important. The latter is determined relative to the first day of the last cycle of chemotherapy or, in the case of HSCT recipients, the day of the HSCT.
To avoid omitting the consideration of other noninfectious causes of fever in neutropenic patients, the clinical evaluation should include questions pertaining to the temporal association of the febrile episode to the administration of blood products, to a history of fever associated with the underlying disease, administration of chemotherapeutic agents or amphotericin B, presence of thrombophlebitis, and the possible association of the febrile episode with thromboembolic or hemorrhagic events. For example, in a series of neutropenic patients undergoing remission-induction therapy for acute leukemia,24 36% of febrile episodes were due to noninfectious causes.
The physical signs of inflammation and infection are influenced by the ANC. The incidence and magnitude of localizing findings such as exudate, fluctuance, ulceration, or fissure formation are reduced in a direct relationship to the ANC.130 Other localizing findings, such as erythema and focal tenderness, appear to remain as useful and reliable signs of infection regardless of the ANC.
The body systems most often involved with infection in neutropenic patients are those associated with integumental surfaces, that is, the upper and lower respiratory tracts, the upper and lower GI tracts, and the skin.107,130,131Table 68-2 lists the pertinent historical and physical clues to be sought in the evaluation of a febrile neutropenic patient.
Clinical Evaluation of the Febrile Neutropenic Patient
| Findings to Be Sought | ||
|---|---|---|
| Body System | Historical Clues | Physical Findings |
| Eye | Blurring of vision | Scleral abnormalities |
| Double vision | Icterus | |
| Loss of vision | Hemorrhage | |
| Pain | Local swelling | |
| Conjunctival abnormalities | ||
| Focal erythema | ||
| Petechiae | ||
| Retina | ||
| Hemorrhage | ||
| “Cotton wool” exudates (eg, candidal endophthalmitis) | ||
| Skin | Skin rash | Central venous catheters |
| Pruritus (focal or diffuse) | Insertion site erythema/pain | |
| History of drug reactions | Tunnel site erythema/pain | |
| Focal pain/swelling | Exit site erythema/pain/exudate | |
| IV catheter site(s) | Peripheral IV catheters | |
| Focal tenderness | ||
| Focal erythema | ||
| Exudate at the insertion site | ||
| Skin rash | ||
| Papular/macular/vesicular morphotypes | ||
| Ulceration | ||
| Focal areas of necrosis | ||
| (eg, ecthyma gangrenosum) | ||
| Distribution | ||
| Upper respiratory tract | Painful ear | External auditory canals |
| Nasal stuffiness | Tympanic membrane erythema | |
| Sinus tenderness | ||
| Epistaxis | ||
| Lower respiratory tract | Cough | Tachypnea (RR >20/minute) |
| Increased volume of respiratory secretions | Tachycardia (HR >90/minute) | |
| Hyperpnea | Localized crepitations | |
| Dyspnea | Effusions (reduced breath sounds) | |
| Hemoptysis | Consolidation (bronchial breathing) | |
| Chest pain | Friction rub | |
| Upper gastrointestinal | Odynophagia | Gingival bleeding |
| Dysphagia | Pseudomembranous exudate over buccal and gingival surfaces and tongue | |
| History of herpes stomatitis | Mucosal erythema | |
| History of denture use | Mucosal ulceration | |
| Focal pain | ||
| Preexisting periodontitis | ||
| Lower gastrointestinal tract | Abdominal pain | Focal abdominal pain |
| Constipation | Right upper quadrant pain(eg, biliary tree) | |
| Diarrhea ± bleeding | ||
| Perianal pain with defecation | Right lower quadrant pain (eg, cecum/ascending colon) | |
| Jaundice | Left lower quadrant pain(eg, diverticular disease) | |
| Perianal abnormalities | ||
| Focal tenderness | ||
| Focal/diffuse erythema | ||
| Fissures | ||
| Ulcerations | ||
| Hemorrhoidal tissues | ||
Examination of the head and neck area should include the optic fundi, the external auditory canals and tympanic membranes, the anterior nasal mucosa, the vermilion border of the lips, and the mucosal surfaces of the oropharynx. The funduscopic examination should look for retinal hemorrhages as evidence of a bleeding diathesis and retinal exudates (often described as “cotton wool”) that would suggest endophthalmitis associated with disseminated candidiasis. Examination of the external auditory canals and tympanic membranes for erythema or vesicular lesions can implicate this as a focus for infection by respiratory pathogens or herpes group viruses. The anterior nasal mucosal surfaces should be examined for ulcerated lesions suggesting the presence of a local filamentous fungal infection such as Aspergillus. The skin of the external nares should be examined for vesicular or crusted lesions suggesting HSV. Nasal stuffiness and maxillary sinus tenderness suggests the presence of sinusitis.
The oropharyngeal examination consists of inspection of the dentition, gingival surfaces, mucosal surfaces of the cheeks, hard and soft palate, tongue surfaces, and posterior pharyngeal wall. The presence of decaying teeth and gingival hyperemia implicates those sites as possible sources of bacteremic infection. The presence of shallow, painful mucosal ulcers on an erythematous base suggests herpes mucositis. Progression of this kind of lesion with local tissue necrosis can suggest a polymicrobial infection due to oropharyngeal anaerobic bacteria (eg, Fusobacterium nucleatum, Bacteroides melaninogenicus, peptostreptococci), particularly if cultures for HSV are negative or if such lesions develop during prophylactic or therapeutic administration of acyclovir. Oral thrush or pseudomembranous pharyngitis evolves from an overgrowth of opportunistic yeasts such as Candida species. These lesions are characterized by a thick creamy pseudomembrane consisting of masses of fungi existing in both the yeast and the mycelial phases. The distribution may be patchy, confluent, or discrete. The pseudomembrane is frequently closely adherent to the underlying mucosal surface such that attempts at removal reveal an erythematous or hemorrhagic base. The diagnosis is suspected by the clinical appearance and confirmed by the demonstration of the pathogen in culture and by the appearance of budding yeasts and pseudohyphae on a Gram stain or KOH preparation.
Chest examination should emphasize evaluation of the lower respiratory tract and central venous catheter sites. The typical signs of pulmonary consolidation may be muted or absent in neutropenic patients; however, localized crepitation often precedes the appearance of pulmonary infiltrates radiologically and thus often represents the earliest (and often only) clue to a developing pneumonia in a neutropenic patient. Purulent sputum is similarly reduced in incidence and amount. The neutropenic patient with a developing pneumonia, therefore, may manifest only as febrile illness associated with an increased respiratory rate and a few localized crepitations, with or without an associated cough or radiologic changes.132 The clinician must search for additional differential diagnostic clues such as the origin of the suspected pneumonia (community or hospital acquired), the tempo of the illness, the association of the illness with other potentially noninfectious factors such as pulmonary edema, exposure to certain chemotherapeutic agents associated with lung injury (bleomycin, busulfan, cytarabine), radiation therapy, pulmonary thromboemboli, pulmonary hemorrhage, or hyperleukocytosis. Chest physical examination can do little to differentiate infectious or noninfectious causes of pulmonary findings, but it can help identify the lower respiratory tract as the potential infected focus.
The symptoms and signs of an intra-abdominal infection may be obvious or muted, focal, or diffuse. The most important finding is focal tenderness.130 For example, tenderness in the right lower quadrant might suggest neutropenic enterocolitis (typhlitis); right upper quadrant tenderness, a biliary tract focus or hepatomegaly; epigastric pain, an upper GI focus; and left lower quadrant tenderness, colitis or diverticular disease. It is important to examine the perianal tissues for signs of excoriation, local erythema, swelling, tenderness, fissure formation, or hemorrhoidal tissues, since this area is frequently the site of major life-threatening infection in neutropenic patients. Digital examination of the rectum is not recommended in neutropenic patients because of the additional risk of tissue damage, bleeding, and infection. A light perianal digital examination, however, can be informative about focal areas of cellulitis without increasing the risk of bacteremic infection.
Examination of the skin should consist of a thorough search for focal areas of pain, swelling, or erythema, especially in association with indwelling vascular access devices. Particular attention should be paid to the venous insertion, tunnel, and exit sites associated with central venous catheters. In contrast, nonspecific local pocket tenderness may be the only clue to infection associated with the totally implantable venous access port-reservoir systems.
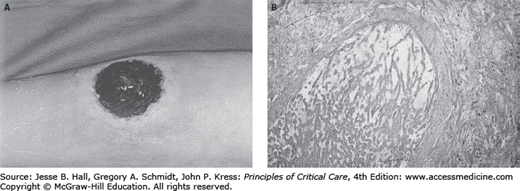
Skin rashes are a common phenomenon among neutropenic patients. The differential diagnosis must include both infectious and noninfectious causes. Among the former group are focal ulcerative and necrotic lesions caused by metastatic pyogenic bacterial infection such as that associated with bacteremic P aeruginosa or Staphylococcus aureus (infections causing ecthyma gangrenosum), or by disseminated angioinvasive filamentous fungi such as that due to Aspergillus species, Scedosporium apiospermum, or Fusarium species (Fig. 68-3A and B). Pustular erythematous lesions diffusely distributed over the skin surface suggest the possibility of disseminated fungal infection such as that caused by Candida tropicalis. Vesicular skin lesions suggest the possibility of infection due to HSV or herpes zoster virus.
FIGURE 68-3
A. Necrotic ulcerated skin lesion in a 53-year-old man on day 15 of remission-induction therapy for AML. This lesion was caused by skin infarction secondary to angioinvasive infection due to Aspergillus flavus.B. Periodic acid-Schiff stain of a biopsy from this lesion demonstrates the invasion of broad, acutely branching septate hyphae into blood vessels.
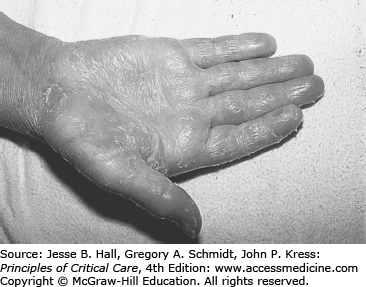
The list of possible noninfectious causes of skin rash is long. The three most important considerations are hemorrhagic petechial or ecchymotic rashes associated with profound thrombocytopenia; hypersensitivity rashes associated with specific drugs such as β-lactam antibacterial drugs, allopurinol, or trimethoprim-sulfamethoxazole (TMP/SMX); and specific chemotherapy regimen–related rash syndromes (eg, the exfoliative palmar/plantar syndrome associated with high-dose cytarabine; Fig. 68-4). These skin rash syndromes may coexist simultaneously.
Once the relevant historical details and physical findings are established, the complete evaluation of the febrile neutropenic patient should include a series of laboratory and radiologic investigations designed to complement the clinical examination. Specimens of body fluids such as blood, urine, cerebrospinal fluid, and lower respiratory secretions should be submitted to the clinical microbiology laboratory for culture and antimicrobial susceptibility testing where appropriate. At least two sets of blood cultures should be obtained, one of which should be from a peripheral venous site. Further, it has been recommended that for patients with multilumen indwelling central venous catheters in situ, each lumen of the catheter should be sampled in addition to blood from the peripheral venous site.120,129
The basic radiologic investigation is the chest radiograph. When suggested by clinical clues, sinus radiographs are useful for detecting sinus opacification or fluid levels. Panorex radiographs can be helpful for evaluating periodontal infection. High-resolution computed tomographic (HRCT) examination of the lungs has a high yield of abnormalities in febrile neutropenic patients despite nondiagnostic chest radiographs.133,134 In one study, 60% of febrile neutropenic patients with normal chest radiographs had a pulmonary infiltrate demonstrable on the chest HRCT.133 Computed tomography (CT) of the abdomen or hepatic ultrasonography is valuable for assessing the significance of abnormalities in cholestatic enzymes (γ-glutamyltransferase [GGT] and alkaline phosphatase). This is particularly important if the possibility of hepatosplenic candidiasis exists. Abdominal pain and tenderness with diarrhea in a persistently febrile neutropenic patient suggests the possibility of neutropenic enterocolitis. Abdominal CT looking for bowel wall thickening, pneumatosis, wall nodularity, mucosal enhancement, bowel dilation, ascites, and mesenteric stranding may be useful.135
Neutropenia-related febrile episodes are heterogeneous with respect to the cause and duration of neutropenia, as well as fever risks and causes. Patients differ in their response to treatment and in their risks of complications. Accordingly, the practice standard has been to hospitalize all febrile neutropenic patients for assessment, empirical broad-spectrum antimicrobial therapy,136 and monitoring for and management of complications. Problems in neutropenic fever include organ failures such as hemodynamic instability (eg, shock, dysrhythmias); respiratory insufficiency; acute kidney injury; pain, nausea, vomiting, and dehydration; delirium; hemorrhage requiring blood product transfusion; changes in metabolic function requiring intervention; and death.
Investigators from the Dana-Farber Cancer Institute137 examined the natural history of febrile neutropenic patients to identify patients at risk for complications due to neutropenia, infection, underlying cancer, and other comorbid conditions. Based on comorbidities and complications, patients could be classified into three groups at high risk for complications and one low-risk group. Group I (39% of the total) comprised hospitalized patients usually with hematologic malignancies or hematopoietic stem cell transplant. Complication and morbidity rates were 34% and 23%, respectively. Group II (8% of the total) comprised outpatients with concurrent comorbidity and had complication and mortality rates of 55% and 14%, respectively. Group III (10% of the total) comprised outpatients with as yet uncontrolled or progressive cancer and had complication and mortality rates of 31% and 15%, respectively. Group IV (the low-risk group, 43% of the total) comprised outpatients with controlled or responding cancer and no comorbid processes. This group had a complication rate of 2% and no deaths. These observations were prospectively validated in follow-up studies.137,138 These results suggest that high-risk patients with characteristics corresponding to groups I to III should be admitted and managed as inpatients with careful monitoring for serious complications, whereas low-risk patients (group IV) can be managed on an outpatient basis.138-146
The Multinational Association for the Supportive Care in Cancer developed and validated a scoring system to identify patients at low risk for serious medical complications that would require admission to hospital.52,53,147 Identifying factors included absence of symptoms; hypotension; airflow obstruction; hematological malignancy; invasive fungal infection; or dehydration. Further, status as an outpatient at the onset of the febrile neutropenic episode and age <60 years were also identifying factors. This system has been offered as a strategy for identifying patients eligible for studies of more cost-effective, safe, outpatient-based management strategies.148
The empirical initial therapy for suspected infection in febrile neutropenic patients is based on three assumptions.
The majority of infections are due to bacteria.149
The principal pathogens are aerobic gram-negative bacilli (E coli, K pneumoniae, and P aeruginosa)107,149, the predominance of gram-positive organisms in blood cultures102,106 notwithstanding.
Inappropriate therapy for aerobic gram-negative bacteremia may be associated with a high mortality150 and a median survival of less than 72 hours.151
Accordingly, empirical first-line therapy regimens are chosen for their activity against these pathogens. The rising prevalence of multidrug resistant (MDR) bacteria152 has required a more critical approach to the choice of initial empirical agents. The increase in infections due to gram-negative bacilli carrying extended-spectrum β-lactamases (ESBL) as well as genes conferring coresistance to other antibacterial classes such as the tetracyclines, fluoroquinolones, and aminoglycosides has led to recommendations for carbapenems as initial treatments in environments where ESBL-producing gram-negative bacteria are prevalent.21 In environments where carbapenemase-producing gram-negative bacterial infections are prevalent, choices are restricted to tigecycline,153 or colistimethate (polymyxin E)154 with poor outcomes in neutropenic cancer patients.155
The clinical assessment may identify features favoring infection by gram-positive organisms,156 warranting additional agents in the initial empirical antibacterial regimen. These predictors include infection sites such as skin and soft tissue or central venous access devices associated with S aureus and coagulase-negative staphylococci; colonization by MDR bacteria that warrant consideration of glycopeptides (eg, vancomycin) for MRSA, oxazolodinones (eg, linezolid), or lipopeptides (eg, daptomycin) for VRE.21,153 Community-acquired pneumonia in a region with high-level macrolide-resistant Streptococcus pneumoniae may also require combination initial therapy.157
Several guidelines panels recommend that febrile neutropenic cancer patients at high risk for medical complications52 be hospitalized for intravenous empirical antibacterial therapy with a single antipseudomonal agent (monotherapy).21,118,129,158,159 While there are circumstances where combination regimens may have an advantage (vide supra and Table 68-4), published evidence does not support routine use of combination regimens containing aminoglycosides160 or glycopeptides in high-risk patients.161 In contrast, guidelines recommend consideration of orally administered combination initial empirical antibacterial therapy (eg, ciprofloxacin and amoxicillin/clavulanate) for low-risk febrile neutropenic patients being considered for outpatient management.21 Tables 68-3 and 68-4 list the commonly used agents and the circumstances where they may be considered.
Antimicrobial Therapy Used for Therapy in Febrile Neutropenic Patients
| β-Lactam Antibiotics | Typical Dosing |
|---|---|
| Ticarcillin + clavulanic acid | 200-300 mg/kg per day IV in 4-6 divided doses |
| Piperacillin + tazobactam | 200-300 mg/kg per day IV in 3-4 divided doses |
| Cefoperazone | 2 g q12h IV |
| Ceftriaxone | 2 g q24h IV |
| Ceftazidime | 2 g q8h IV |
| Cefepime | 2 g q8h IV |
| Imipenem/cilastatin | 500 mg q6h IV |
| Meropenem | 1 g q8h IV |
| Aminoglycosides | |
| Gentamicin | 1.5-2 mg/kg q8h IV |
| Netilmicin | 1.5-2.0 mg/kg q8h IV |
| Tobramycin | 1.5-2 mg/kg q8h IV |
| Amikacin | 7.5 mg/kg q12h IV |
| Fluoroquinolones | |
| Ciprofloxacin | 400 mg q12h IV |
| 500-750 mg q12h PO | |
| Levofloxacin | 500-750 mg q24h PO/IV |
| Norfloxacin | 400 mg q12h PO |
| Moxifloxacin | 400 mg Q12h PO/IV |
| Macrolides | |
| Erythromycin | 0.5-1.0 g q6h IV |
| Azithromycin | 500 mg IV/PO day 1, then 250 mg IV/PO q24h |
| Glycopeptides | |
| Vancomycin | 1.0 g q12h IV or 30 mg/kg IV q24h |
| Teicoplanin | 800 mg IV day 1, then 400 mg IV q24h |
| Dalbavancin | 1 g IV day 1, then 500 mg IV q7days |
| Other antibacterial agents | |
| TMP-SMX | 10-20 mg/50-100 mg/kg per day in 4 divided doses |
| Metronidazole | 500 mg q8h IV/PO |
| Linezolid | 600 mg q12h IV/PO |
| Daptomycin | 4-6 mg/kg/24 hours IV |
| Antiviral agents | |
| Acyclovir | HSV: 400 mg 5 times daily, or 5 mg/kg q8h IV |
| VZV: 800 mg 5 times daily PO, or 10 mg/kg q8h IV | |
| Valacyclovir | HSV: 500 mg q12h PO |
| VZV: 1000 mg q8h PO | |
| Famciclovir | HSV: 500 mg q12h PO |
| VZV: 750 mg q24h or 500 mg q12h PO | |
| Ganciclovir | 5 mg/kg q12h IV |
| Valganciclovir | 900 mg q12h PO |
| Polyene antifungal agents | |
| Amphotericin B deoxycholate | 0.5-1.0 mg/kg per day IV |
| Amphotericin B lipid complex | 5 mg/kg per day IV |
| Liposomal amphotericin B | 3-5 mg/kg per day IV |
| Triazole antifungal agents | |
| Fluconazole | 200-400 mg IV/PO q day |
| Itraconazole | 200-400 mg PO q day |
| Voriconazole | 6 mg/kg IV q12h day 1, then 4 mg/kg q12h IV, or 200-300 mg PO q12h |
| Posaconazole | 200 mg q8h PO |
| Echinocandin antifungal agents | |
| Caspofungin | 70 mg IV day 1, then 50 mg per day IV |
| Micafungin | 100 mg q24h IV |
| Anidulafungin | 200 mg IV day 1, then 100 mg q24h IV |
| Other antifungal agents | |
| 5-Fluorocytosine | 150 mg/kg per day PO in 4 divided doses |
| Terbinafine | 250 mg q8h PO |
Considerations Governing the Choice of Empiric Antibacterial Regimen
| β-Lactam/β-lactamase inhibitora monotherapy, or carbapenemb monotherapy | High-riskc neutropenic fever syndromes |
| Aminoglycosided monotherapy | Not recommended |
| β-Lactam ± β-lactamase inhibitora + a fluoroquinolonee or aminoglycosided | High-riskc neutropenic fever with severe sepsis/septic shock |
| Risk of MDRf gram-negative bacilli such as P aeruginosa, E coli, K pneumoniae, Enterobacter spp, Acinetobacter spp, Stenotrophomonas spp | |
| Severe sepsis or septic shock syndromes | |
| Tigecycline or colistimethate | Risk of metallocarbapenemaseg-producing Gram-negative bacillary infection |
| Monobactamh or third-generation cephalosporini + vancomycin | Patients with known penicillin hypersensitivity |
| High-riskc neutropenic episodes | |
| Oral therapy: fluoroquinolonee + a β-lactam/β-lactamase inhibitora | Short-term neutropenic episodes (ANC <0.5 × 109/L, <7 days) |
| Low-riskc neutropenic episodes | |
| Other gram-positive active agents: | |
| Vancomycin | Suspected or proven coagulase-negative staphylococcal infection |
| Suspected vascular catheter infection | |
| Skin or soft tissue infection | |
| Suspect MRSAj | |
| Hemodynamic instability/severe sepsisk | |
| Pneumoniak | |
| Linezolid or daptomycin | Suspect MRSAj or VREl |
| Metronidazole | Suspect intra-abdominal infection |
| Necrotizing gingivitis | |
| Severe oral mucositis | |
| Suspect perianal infection | |
| Proven Clostridium difficile–associated diarrhea |
Combination regimens include two β-lactam agents26,162,163, combined with aminoglycosides107-109,111,112,114,115 or combined with fluoroquinolones.106,164,165 Single-agent regimens consist of β-lactam agents113-115,117,166-169 with or without β-lactamase inhibitors (tazobactam, clavulanic acid, or sulbactam) or fluoroquinolones.170-172 Monotherapy with aminoglycosides is not recommended.120
β-lactam antibacterial agents may be categorized as extended-spectrum antipseudomonal penicillins (eg, carbenicillin, ticarcillin with or without clavulanic acid, piperacillin with or without tazobactam, azlocillin, or mezlocillin), third- or fourth-generation antipseudomonal cephalosporins (eg, moxalactam, ceftriaxone, ceftazidime, cefoperazone with or without sulbactam, cefpirome, or cefepime), or as carbapenems (eg, imipenem/cilastatin, meropenem, or ertapenem). The addition of β-lactamase inhibitors enhances spectrum of activity against β-lactamase-producing bacteria.112,116,173-191
In a review of prescribing behavior for 214 febrile neutropenic patients in Canadian centers, single-agent initial empirical therapy was administered in 42% of cases (third-generation cephalosporin—32%, carbapenem—2.3%, fluoroquinolone—0.9%).192 Combination therapy was administered in 58% of cases (antipseudomonal penicillin plus aminoglycoside—29%, antipseudomonal cephalosporin plus aminoglycoside—15%, antipseudomonal β-lactam plus glycopeptide—11%).192 Vancomycin was part of the initial empirical antibacterial therapy in 15% of cases. First modification with second-line therapy for persistent fever was administered in 87% of the cases after a median of 5 days. Empiric amphotericin B was administered for persistent fever in 48% of cases after a median of 9 days. Previous studies have demonstrated that glycopeptides are used as empirical second-line therapy in 40% to 50% of cases after first-line empiric therapy with extended spectrum cephalosporins.113,116,174-177,193-204
The Role of Aminoglycosides: Aminoglycosides have been part of the standard combination empirical antibacterial therapy for the management of febrile neutropenic patients from the early 1970s to the 1990s. The combination of an aminoglycoside with an antipseudomonal β-lactam antibacterial agent was designed to provide a broad spectrum of antibacterial activity, achieve bactericidal serum concentrations, exert a synergistic antibacterial effect, and prevent emergence of resistance. Such combinations have been recommended in the published guidelines by the Infectious Diseases Society of America,65,120,136 the National Comprehensive Cancer Network,205,206 and the Infectious Diseases Working Party of the German Society of Hematology and Oncology118 but not the Spanish guidelines.207 The choice of aminoglycoside must be based on bacterial susceptibility patterns, availability of serum aminoglycoside concentration monitoring, and drug cost.
A large randomized controlled trial (N = 733) compared piperacillin/tazobactam to piperacillin/tazobactam plus amikacin.116 The primary outcome was defervescence of all signs and symptoms of infection without modification of the initial antibacterial regimen. Response was observed in 49% monotherapy versus 53% combination recipients (p = 0.2). The response rates in single pathogen gram-positive bacteremias were low (27% and 32%, respectively) because of the high proportion of coagulase-negative staphylococcal bacteremias. In contrast, the response rates for streptococcal and enterococcal bacteremias between the two groups were significantly higher (60% and 71%, respectively, p = 0.7). The response rates for single gram-negative bacteremias were also similar (36% and 34%, respectively; p = 0.9). The aminoglycoside failed to enhance the response rates in any circumstance. The overall mortalities in the monotherapy and combination therapy groups were 4% and 6%, respectively (p = 0.2).
Two systematic reviews of the literature have examined the safety and efficacy of β-lactam plus aminoglycoside combinations in febrile neutropenic patients in comparison to monotherapy.160,208 Furno et al reviewed 4795 heterogeneously treated febrile neutropenic episodes from 29 randomized controlled clinical trials comparing monotherapy (ceftazidime, 9 trials; cefepime, 2 trials; cefoperazone, 1 trial; imipenem/cilastatin, 9 trials; meropenem, 4 trials; ciprofloxacin, 2 trials; ofloxacin, 2 trials) and aminoglycoside-based combination therapy. The pooled odds ratios for overall treatment failure and for treatment failure in bloodstream infections were significant at 0.88 and 0.70, respectively, demonstrating fewer failures in the monotherapy groups.160 Paul et al examined 7807 febrile neutropenic patients entered into 47 randomized controlled trials comparing β-lactam monotherapy to β-lactam plus aminoglycoside combination therapy.208 The main outcome was overall mortality. While there was no significant difference in overall mortality (7.8% vs 9.1% for monotherapy and combination therapy, respectively; RR 0.85; p = 0.08), there were fewer failures among β-lactam monotherapy recipients.208 Monotherapy recipients had fewer adverse events overall and less nephrotoxicity.208 On the basis of these analyses, β-lactam plus aminoglycoside combinations appear to offer no advantages over broad-spectrum β-lactam-based monotherapy. Further, the combination regimens present significant disadvantages with respect to toxicity and costs related to drug monitoring and administration.
Despite these observations, guidelines recommend consideration of aminoglycoside combination therapy for seriously ill neutropenic cancer patients with hypotension or pneumonia.21 A systematic review of randomized or observational studies in patients with serious bacterial infections observed a survival benefit of combination therapy among patients with severe sepsis or septic shock where the mortality risk associated with monotherapy was >25% (OR 0.88; p = 0.0447).209 Where the risk of death among monotherapy recipients was ≤15%, mortality was higher among combination therapy recipients (OR 1.53; p = 0.003). This effect was confirmed in a retrospective propensity matched multicenter cohort study in which timely (within the first hour of shock) administration of the combination of the β-lactam agent with an aminoglycoside, fluoroquinolone, or a macrolide was associated with the lowest 28-day mortality.210 The all-cause mortality risk among febrile neutropenic patients receiving β-lactam monotherapy or β-lactam plus aminoglycoside-based combination therapy reported in clinical trials has been <10%.208 These observations may explain the apparent discordance between these meta-analyses208,209 and support the IDSA recommendations for restricting combination therapy to neutropenic fever associated with severe sepsis or septic shock where the mortality risk is very high, and for administering monotherapy for patients who are hemodynamically stable.
Fluoroquinolones in the Treatment of Febrile Neutropenic Patients: The fluoroquinolones evaluated in studies of the empirical treatment of febrile neutropenic patients include ciprofloxacin,106,211 perfloxacin,212 ofloxacin,140,141 levofloxacin,213 clinifloxacin,170,172 and moxifloxacin.214,215 These agents have the advantage of availability in both oral and intravenous formulations.211,216,217
Studies of empirical fluoroquinolones as first-line therapy for febrile neutropenic patients have largely targeted those patients at lower risk for medical complications. Ciprofloxacin with and without other agents such as clindamycin, aztreonam, or amoxicillin has been the most completely studied.140,142,143,145,185,218-222 The administration of ciprofloxacin 750 mg orally and amoxicillin/clavulanate 625 mg orally—both every 8 hours—was well tolerated and as effective as intravenous ceftriaxone plus amikacin218 or ceftazidime219 administered on an inpatient basis. Similar results have been reported for trials comparing oral ciprofloxacin-based regimens and intravenous regimens on an outpatient basis. These strategies appear to be safe and effective for low-risk patients. A recent survey of 1207 physician members of the American Society of Clinical Oncology demonstrated that 82% of these physicians prescribed outpatient antibacterial therapy for low-risk neutropenic fever patients.223
The National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology have included ciprofloxacin plus an antipseudomonal penicillin as an alternative empiric regimen for higher neutropenia.224 One large trial compared piperacillin plus ciprofloxacin to piperacillin plus tobramycin in intermediate- to high-risk febrile neutropenic cancer patients.106 Success rates (ie, defervescence without initial regimen modification) were similar; however, times to defervescence were faster among the piperacillin/ciprofloxacin recipients, 5 versus 6 days (p = 0.005).
Reports of gram-negative fluoroquinolone resistance in neutropenic cancer patients began to emerge in the early 1990s.225-227 The incidence of fluoroquinolone-resistant Escherichia coli (FREC) bacteremia among patients treated on the EORTC-IATCG clinical trials from 1983 to 1993 increased from zero (1983-1990) to 28% (1991-1993).225 However, Pseudomonas aeruginosa and Klebsiella pneumoniae resistance remained largely unchanged at less than 10%.225 This has been more of a problem in institutions with rates for gram-negative bacillary fluoroquinolone resistance of over 10% despite community-related resistance prevalence of less than 1%.228 Several investigators have reported high fluoroquinolone resistance rates.227,229
Fluoroquinolone resistance among community-derived gram-negative bacilli, and FREC in particular, has emerged in parallel with the increased prescribing of these products in the community.230-232 There is a significant correlation between the incidence of ciprofloxacin-resistant Escherichia coli bloodstream infection and the increased community and hospital use of fluoroquinolones.230 Among those who had not heretofore received fluoroquinolone therapy, Garau and colleagues reported the prevalence of FREC in the stool sample to be approximately 25%.232 These investigators also observed a very high prevalence of FREC in the stools of pigs and poultry and argued that the increased prevalence of human carriage of FREC may be linked to the high prevalence in animal-based food products. The increased use of fluoroquinolones in animal feeds and in humans is believed to play a role in the selection for FREC. In an environment with a high prevalence of gram-negative fluoroquinolone resistance, patients receiving ciprofloxacin chemoprophylaxis while undergoing high-dose chemotherapy with stem cell rescue became colonized with FREC in one-third of cases,233 increasing the likelihood of FREC bloodstream infections.234
Inappropriate use of fluoroquinolones is common. A case:control study of fluoroquinolone use in emergency departments demonstrated inappropriate prescription of these agents in 81% of cases.235 Lastly, coresistance gram-negative bacilli to fluoroquinolones and other antibacterials is being reported more frequently.232,236 Inappropriate use of fluoroquinolones in the community is strongly linked to resistance and reduces the likelihood that this class of agents will be useful.225,227
Double β-lactam Combinations: Regimens consisting of an antipseudomonal broad-spectrum β-lactam plus an extended spectrum third-generation cephalosporin are safe and effective alternatives to β-lactam plus aminoglycoside regimens26,163,237; however, they are costly and do not offer any advantages over broad-spectrum β-lactam/β-lactamase inhibitor monotherapies. These regimens may have an occasional role with preexisting renal insufficiency, where the patient is receiving concomitant nephrotoxic agents (eg, cyclosporine or cis-platin) or where gram-positive organisms such as viridans streptococci are suspected.26,93 The availability of effective β-lactam–fluoroquinolone or β-lactam monotherapy-based strategies has largely rendered double β-lactam regimens obsolete.21
Extended-Spectrum Cephalosporins: The intrinsic activity of many of the third- and fourth-generation cephalosporins against aerobic gram-negative bacilli is high. These agents have been used effectively as single agents for empirical treatment of suspected infection.107-115,169,238 Empirical monotherapy has been shown to be effective in both low- and high-risk febrile neutropenic patient populations and in those whose expected duration of severe neutropenia (ANC <0.5 × 109/L) is either longer or shorter than 7 days. Experience suggests that single-agent empirical regimens require modification in one-third to one-half of patients with neutropenic periods in excess of 1 week.111,113-115 There is a lower likelihood that modifications will be necessary with short-term (<1 week) neutropenia.
A meta-analysis examining the efficacy of ceftazidime monotherapy compared to standard combination therapy for the empirical treatment of febrile neutropenic patients failed to demonstrate a difference in the odds of overall treatment failure and failure in bloodstream infections.169 Another meta-analysis238 compared the efficacy of ceftriaxone with (7 trials) or without (1 trial) an aminoglycoside and ceftazidime with (6 trials) or without (1 trial) an aminoglycoside or azlocillin plus an aminoglycoside (1 trial). There were no differences in any outcomes, including mortality, noted in these comparative analyses. These analyses demonstrate that empirical antibacterial therapy of febrile neutropenic patients with once-daily ceftriaxone is as effective as thrice daily ceftazidime. This has important implications for potential outpatient once-daily intravenous therapy.
Carbapenems for Treatment of Febrile Neutropenic Patients: Both imipenem/cilastatin and meropenem have been studied widely as empirical therapy in febrile neutropenic patients. A meta-analysis examined the efficacy of imipenem/cilastatin compared to a β-lactam plus aminoglycoside (11 trials) and to β-lactam monotherapy (ceftazidime, 4 trials; cefoperazone/sulbactam, 1 trial) or β-lactam plus glycopeptide (ceftazidime, 3 trials) or double-β-lactam therapy (cefoperazone/mezlocillin, 1 trial; cefoperazone/piperacillin, 1 trial). There were fewer treatment failures among imipenem/cilastatin recipients compared to β-lactam plus aminoglycoside combinations or to non-aminoglycoside-containing regimens.168 These analyses support the superiority of imipenem/cilastatin over control arms largely based on third-generation cephalosporins.
Another meta-analysis comparing carbapenem monotherapy to β-lactam plus aminoglycoside combination therapy demonstrated fewer treatment failures among with carbapenems160 in contrast to antipseudomonal cephalosporin monotherapy. A similar analysis of the published clinical trials of meropenem monotherapy compared to ceftazidime-based regimens with or without an aminoglycoside demonstrated greater response rates among meropenem recipients.239 Such observations suggest the inferiority of third-generation cephalosporin monotherapy regimens compared to carbapenem monotherapy.
Piperacillin/Tazobactam for the Treatment of Febrile Neutropenic Patients: Piperacillin/tazobactam plus amikacin therapy was successful in 61% of febrile neutropenic patients studied by the European Organisation for the Research and Treatment of Cancer compared to 54% of patients receiving ceftazidime plus amikacin (p = 0.05).112 Furthermore, the time to defervescence was shorter among piperacillin/tazobactam recipients (p = 0.01) and the frequency with which the initial regimen was modified by the addition of a glycopeptide was lower (24% vs 35%; p = 0.002). Another large Italian trial examined the role of the aminoglycoside, amikacin, when combined with piperacillin/tazobactam. 116 The response rates overall between the two groups were similar regardless of the classification of the febrile neutropenic episode as bacteremic, clinically documented, or unexplained fever. Clearly, aminoglycosides offered no advantage over piperacillin/tazobactam monotherapy. Piperacillin/tazobactam monotherapy has been studied compared to extended spectrum cephalosporins with or without aminoglycosides.173-177 Overall unmodified success rates were significantly higher among piperacillin/tazobactam recipients.177 Further, the frequency of second-line glycopeptide therapy was lower with piperacillin/tazobactam.177 These observations demonstrate the superiority of initial piperacillin/tazobactam monotherapy over extended spectrum cephalosporins with or without aminoglycosides. Further, the need to modify the initial antibacterial regimen by the addition of a glycopeptide (eg, vancomycin) for persistent fever is also significantly less than for the cephalosporin-based comparator groups. This has important implications with respect to cost, drug-related toxicities, and for selection of resistant microorganisms (eg, vancomycin-resistant Enterococcus).
The Role of Glycopeptides in Febrile Neutropenic Patients: For more than a decade, gram-positive bacteria have dominated as pathogens in microbiologically documented infections observed in neutropenic patients. Two decades ago approximately 70% of bacteremic isolates were gram-negative bacilli, whereas more recently approximately 60% to 70% are gram-positive cocci.102,106,156 Almost 30 years ago, a ceftazidime-based empirical trial observed more fatal gram-positive superinfections than expected.240 Subsequently, a small study demonstrated that the addition of vancomycin to ceftazidime therapy reduced the superinfection rate from 24% to zero, and the infection-related mortality rate by 91%.241 Such observations have led investigators to advocate the inclusion of glycopeptides as part of the first-line empirical antibacterial therapy in febrile neutropenic patients.
In contrast, two studies from the National Cancer Institute suggested that the vancomycin therapy may be safely delayed without increased morbidity or mortality.114,242 In order to examine this question further, the European Organization for Treatment and Research in Cancer and the National Cancer Institute of Canada Clinical Trials Group conducted a large randomized trial comparing empirical therapy with ceftazidime plus amikacin (CA) and ceftazidime plus amikacin plus vancomycin (CAV).110 The overall response rate was 76% among CAV recipients versus 63% among CA recipients (p < 0.001), the difference largely due to differential response rates for gram-positive bloodstream infections among CAV recipients. Despite the apparent superiority of the CAV arm, persistently febrile CA patients at day +3 received vancomycin resulting in no differences in overall success rates or mortality. Treatment failures were due to lack of prompt response and to persistent fever very early after the initiation of the allocated regimen rather than objective indicators of failure (eg, persistence of resistant pathogens or progression at foci of infection). Patients receiving CA were more likely to receive vancomycin with persistence of fever at day +3 than recipients of the CAV regimen.243 Since the median time to defervescence among high-risk febrile neutropenic patients is day 5106,112,113,115,204 many were considered failures unnecessarily because of regimen modification before they would have had a chance to defervesce. Compulsion to modify the initial antibacterial regimen for persistent fever at day +3 is driven, in part, by previously published protocols114 reinforced by previously published guidelines.120,136
Three other trials244-246 examining the role of initial glycopeptide therapy in febrile neutropenic cancer patients came to similar conclusions as the EORTC/NCIC trial110; that is, the inclusion of glycopeptides as initial therapy results in modest improvements in response rates, particularly with gram-positive bacteremia, but has no impact on overall survival of the neutropenic episode. Second-line glycopeptide-based empirical antibacterial therapy for persistent fever has become quite common. As noted above, almost half of cases enrolled in clinical trials have a glycopeptide administered for these reasons.113,116,174-177,193-204 The efficacy of empiric second-line glycopeptide therapy has been studied in two randomized controlled trials.178,247 Both studies failed to demonstrate a significant treatment effect with regard to defervescence or overall mortality compared to placebo.248 Three systematic reviews with meta-analyses of this subject have concluded independently that glycopeptides should not be used routinely for the initial empirical regimen.161,248,249 These observations have led to recommendations by the American, German, and Spanish guideline panels that glycopeptides not be used as part of the initial empirical regimen for neutropenic fever unless there is evidence of gram-positive infection.21,65,118,207,250
The use of glycopeptides as part of the initial first-line regimen should be reserved for patients at highest risk for serious gram-positive infection.21,65,224 Such circumstances include clinical catheter-related infection, infection in patients receiving fluoroquinolone-based antibacterial chemoprophylaxis associated with severe mucositis predisposing patients to viridans group Streptococcal bloodstream infections,97,98,251 infection in the setting of colonization by methicillin-resistant Staphylococcus aureus (MRSA), bloodstream isolate characterized as gram-positive cocci in groups and clusters (suggesting Staphylococcus spp and the likelihood of a methicillin-resistant coagulase-negative Staphylococcus), and the setting of septic shock without an identified pathogen. The caveat is that the glycopeptide antibiotic should be discontinued in 2 to 3 days if a resistant gram-positive infection has not been identified.
The Role of Hematopoietic Growth Factors in the Management of Febrile Neutropenic Patients: The inclusion of hematopoietic growth factors (HGF), granulocyte (G-CSF), and granulocyte-macrophage (GM-CSF) colony-stimulating factors in strategies for the prevention and management of febrile neutropenic patients remains unsettled.252 In an older retrospective study in which 30 unstable febrile neutropenic patients given HGF upon admission to the ICU were compared to 30 similar patients not given HGF, there were no detectable treatment effects upon the duration of neutropenia, the length of ICU stay, or overall survival.253 Berghmans and colleagues published a meta-analysis to critically examine the evidence for use of these products in the treatment of febrile neutropenia.254 Eleven studies encompassing 1218 febrile neutropenic episodes published between 1990 and 1998 were considered. Although six trials reported treatment effects for mortality, overall there was no effect on mortality. Further, there were no differences when analysis was conducted by G-CSF versus GM-CSF. The impact of HGF on length of hospitalization was decreased in four trials, unaffected in four trials, and not reported in three trials. Only three of six trials reporting on the impact of HGF on duration of antibacterial therapy demonstrated a reduction in this outcome. In nine trials wherein the impact of HGF on the duration of fever was reported, no treatment effects were observed in seven trials, fever reduction was observed in one trial, and no analysis was provided in one other. The studies included in this analysis were flawed by the failure to control for important variables including the duration and intensity of neutropenia, type of cytotoxic therapy, and type of malignancy. On the basis of this review, Berghmans and colleagues could not recommend routine use of HGF in the treatment of febrile neutropenic episodes.254
American Society of Clinical Oncology guidelines do not recommend HGF use as in neutropenic fever with suspected infection.255,256 However, most do not follow these guidelines, as usage seems to be influenced more by reimbursement than evidence.257-259,260
HGF have been evaluated in other nonneutropenic patient populations including community-acquired pneumonia, human immunodeficiency virus infection, neonatal sepsis, diabetic foot ulcer infections, acute hepatic failure or cirrhosis, orthotopic liver transplantation, and critical care. While there appears to some promising results in some subgroups of patients, HGF have no proven benefit in nonneutropenic critically ill patients with regard to morbidity or mortality.261
Considerations in the Assessment of Initial Empirical Antibacterial Therapy-related Treatment Effects: Response to the initial empirical antibacterial regimen has been defined by defervescence of neutropenic fever, and resolution of the associated signs and symptoms of infection. In unexplained neutropenic fever, defervescence may be the only objective sign by which to gauge response. In clinical trials of empirical antibacterial therapy of high-risk febrile neutropenic patients, defervescence followed by the maintenance of an apyrexial state for 4 to 5 consecutive days has been used as a common definition of response. Defervescence plus resolution of baseline clinical foci of infection have been the major criteria for response in clinically documented infections. Defervescence, resolution of the baseline signs and symptoms associated with the focus of infection, and eradication of the pathogen have been the criteria that define response for microbiologically documented infections.
Treatment success, defined as above, and without modification of the initial empirical antibacterial regimen, has been reported in systematic reviews to be of the order of 60%.208 Another recent meta-analysis was able to demonstrate lower all-cause mortality rates among febrile neutropenic patients receiving piperacillin/tazobactam as compared to other agents.262 In that same review, carbapenems were associated with similar all-cause mortality rates as other antibacterial regimens; lower failure rates and lower rates of antibacterial regimen modifications were observed for the carbapenems. Of note, carbapenems have been associated with a higher risk for Clostridium difficile–associated diarrhea.262
The correctness of the initial empirical choice of antibacterial regimen has an important impact upon outcome in sepsis.150 Some environments with a high prevalence of multidrug-resistant (MDR) bacteria confound the clinician’s initial empirical choice.263 In circumstances where the neutropenic fever is due to a bacterium resistant to the empirical β-lactam, the rates of clinical success have been significantly lower, at less than 10% and with higher all-cause mortality rates of approximately 25%.264 Carbapenems offer broad-spectrum activity against ESBL-producing gram-negative bacilli and may have an advantage where such organisms are prevalent.265
There are several patterns by which failure of the initial empirical antibacterial therapy to resolve the neutropenic fever is recognized. The International Antimicrobial Therapy Cooperative Group of the EORTC defines failure in several ways including death due to the primary infection; persistence of bacteremia beyond the first 24 hours of therapy; breakthrough bacteremic infection while receiving the initial regimen; resistance to the initial β-lactam agent independent of the patient’s condition; failure to defervesce after the first 72 hours of initial therapy; modification of the initial empirical regimen within the first 72 hours for reasons of shock, multiorgan damage, ARDS, or progression of the primary infection.113 Based on these clinical trial-based definitions of failure, a number of syndromes may be recognized.
First, patients may experience a persistence of the initial neutropenic fever (PNF).266 This occurs in 20% of cases.
Second, there may be progression of the primary infection requiring modification of the initial empirical regimen. This occurs in about 15% of neutropenic fevers and may be manifested by isolation of a pathogen-resistant to the empiric β-lactam (the majority are coagulase-negative staphylococci), progression of a clinical focus, persistent bacteremia despite antibacterial therapy, or severe sepsis/septic shock113
Third, patients may develop a recrudescent neutropenic fever266 after an initial defervescence (∼15% occurrence).112,113,267-269 Thirty percent of these are clinically documented infections, 40% are microbiologically documented, and 30% are unexplained.269
Fourth, treatment-related toxicities may lead to discontinuation of the initial empirical therapy. This is uncommon among the β-lactam-based regimens (0.3%-3.2%).262 Discontinuance due to nephrotoxicity among aminoglycoside-containing regimens occurs in 5.5% of cases.208
Fifth, patients may die during treatment of their neutropenic fever. This is relatively rare, ranging from 3.3% for piperacillin/tazobactam regimens to 6.1% for other β-lactam regimens.262
Modification of the initial empirical regimen, regardless of the reason, is a common reason cited for failure of the initial regimen in clinical trials. One of the most common modifications has been the addition of a glycopeptide antibiotic. This occurs in 20% to 33% of cases.262 In one trial, glycopeptides constituted 88% of the modifications to the initial regimen.177 Some of these modifications have been deemed unnecessary, particularly if for persistent fever alone without clear evidence of gram-positive infection.21,113 Widespread vancomycin use has been linked to colonization and infection by vancomycin-resistant Enterococcus
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree