Approach to Bacterial Skin Infections
Peter C. Schalock
Arthur J. Sober
Part 1: Cellulitis
Cellulitis represents bacterial infection of the skin that involves the deeper subcutaneous layers. It must be differentiated from inflammatory skin changes caused by vascular insufficiency and phlebitis. Once cellulitis is identified, the primary physician needs to decide whether the patient can be managed at home on oral antibiotics or requires hospitalization. Prompt recognition and treatment of community-acquired methicillin-resistant staphylococcal skin infection (MRSA) is becoming a particularly important challenge.
Pathogenesis
Any process that compromises the integrity of the skin allows normal skin bacteria access to the underlying subcutaneous tissue, which may initiate an inflammatory response. Trauma, stasis ulceration, ischemia, and chronic edema are common precipitants. On the lower extremity, tinea pedis may allow a site of entry. Contiguous or hematogenous spread from other sites occurs uncommonly.
Organisms and Settings
The organisms most commonly producing cellulitis are normal skin flora, with Streptococcus pyogenes and Staphylococcus aureus predominating. Haemophilus influenzae has been becoming less prevalent since the widespread use of the Hib vaccine.
Methicillin-resistant Staphylococcus aureus (MRSA) strains are increasingly identified in community-acquired cases of cellulitis, now accounting for most cases of skin infection seen in local emergency rooms. Although the majority of community-acquired disease still derives from health care facilities, outbreaks of community-acquired MRSA infection distinct from that associated within health care facilities have been reported among all age and socioeconomic groups, including members of contact-sports teams and attendees of child care centers. Risk factors are incompletely understood but are believed to be related in part to the high frequency of excessive antibiotic exposure (e.g., use in foods, soaps, and treatment of viral illnesses). Most cases seen involve just the skin and soft tissue, but potentially fatal invasive disease may ensue (the reported incidence is about 5%); many strains produce the dermonecrotic cytotoxin known as Panton-Valentine leukocidin.
Staphylococci also produce disease through their ability to multiply and produce a host of extracellular enzymes, including α- and β-hemolysin, leukocidin, coagulase, hyaluronidase, and lipases. Streptococci produce more than 20 extracellular enzymes.
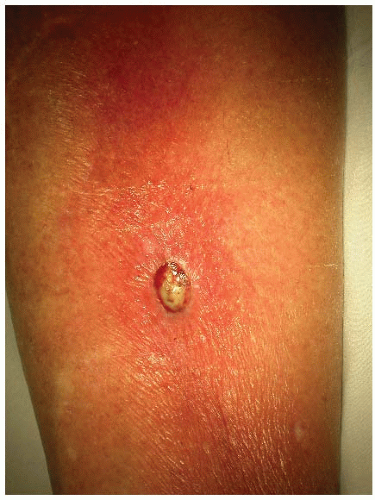
Conditions that impair the host response may predispose to skin infection by opportunistic organisms such as gram-negative bacteria. Skin infections with Escherichia coli, Pseudomonas, and Klebsiella are seen in immunosuppressed patients, diabetics, and alcoholics (Fig. 190-1). Cellulitis in the perineum may be caused by enteric aerobic and anaerobic bacteria.
Injury to mucosal surfaces predisposes to infection with anaerobic organisms. Anaerobic bacteria can produce hyaluronidase, proteases, neuraminidase, and extracellular enzymes. They may act synergistically with aerobic bacteria. Anaerobes play an important role in diabetic foot ulcers, abscesses, and traumatic
wounds. Anaerobic infection also occurs in the setting of crush injuries. The degree of pain may be disproportionate to skin findings. A mixture of aerobic and anaerobic streptococci can cause fasciitis and cellulitis. Once connective tissue is involved, infection spreads along fascial planes.
wounds. Anaerobic infection also occurs in the setting of crush injuries. The degree of pain may be disproportionate to skin findings. A mixture of aerobic and anaerobic streptococci can cause fasciitis and cellulitis. Once connective tissue is involved, infection spreads along fascial planes.
In certain settings, cellulitis is caused by unusual organisms. In persons who handle fish, poultry, or meat, cellulitic infection with Erysipelothrix rhusiopathiae may occur. In patients with water-related injury and sometimes in those with immunosuppression, Aeromonas hydrophila can cause cellulitis. Animal bites or scratches are associated with cellulitis caused by Pasteurella multocida and Capnocytophaga canimorsus. Vibrio species have been implicated in salt water-related injuries. Arthropod bites are portals of entry for conventional streptococcal or staphylococcal species, but one must also consider the unusual spreading cellulitis, a reaction to the toxin of a brown recluse spider or a fire ant. Another unusual cause of cellulitis simulating a septic thrombophlebitis is Campylobacter infection, often in the context of concurrent enteritis.
Clinical Presentation
Cellulitis presents with local redness, heat, swelling, and tenderness that develops over a few days. Children usually present with cellulitis of the head and neck, whereas the extremities are commonly affected in adults. Fever, chills, and rigors may precede the cellulitis and herald possible bacteremia. The clinical presentation does not allow delineation of the specific microbial etiology. Red streaks extending proximally in conjunction with tender lymph nodes indicate an associated lymphangitis. Crepitus indicates gas production and suggests anaerobic involvement. Severe infections may be associated with bullae, pustules, or necrotic tissue.
Invasive strains of group A streptococci (M types 1 and 3) cause not only the usual forms of invasive streptococcal disease (scarlet fever, erysipelas, necrotizing fasciitis, myositis) but also a toxic shock-like syndrome associated with mucosal or cutaneous infection. Mortality is as high as 30%. Onset is abrupt, with fever, diarrhea, rigors, and pain. In many cases, pain out of proportion to the presentation can be a clue to the diagnosis. Bacteremia may be detected in 60% of cases; hematogenous spread is common.
There are no distinguishing clinical features of skin and soft tissue infections caused by MRSA that help to differentiate them from skin infections caused by methicillin-susceptible S. aureus.
Cellulitis needs to be distinguished from other causes of focal erythema, swelling, and tenderness, particularly of the lower extremities. Stasis dermatitis is commonly mistaken for cellulitis because both produce erythema of the lower extremity and develop in persons with severe venous insufficiency; however, cellulitis is rarely bilateral in the lower extremities, whereas stasis dermatitis is commonly bilateral. Superficial thrombophlebitis may present similarly, but the inflammatory response is usually centered about the involved vein, which is tender and palpable. Moreover, crusting and scaling are also rare with cellulitis but common with stasis dermatitis, which has an eczematous pathophysiology. Severe arterial insufficiency may also cause a red lower extremity, but the erythema is typically dependent (dependent rubor), pulses are diminished or absent, and the leg is nontender and cold. Lipodermatosclerosis, a consequence of chronic venous insufficiency and recurrent stasis dermatitis, can mimic cellulitis in causing an acutely tender red lower extremity; it is differentiated by chronic swelling in an obese person with an “inverted champagne bottle” appearance to the leg (diameter of leg sharply decreases from knee to ankle) due to chronic scarring—the skin feels thickened and bound down. Lymphedema may predispose to cellulitis, but may be associated with painless redness in the absence of infection in conjunction with its characteristic chronic nonpitting edema of the lower extremity in an obese person.
TABLE 190-1 Differential Diagnosis of Cellulitis | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||
Other skin lesions mimicking cellulitis include erythema nodosum, early Lyme disease, allergic contact dermatitis, eosinophilic cellulitis, and papular urticaria. Erythema nodosum is indicated by lesions that are typically multiple, exquisitely tender, and often pretibial in location. It is a secondary phenomenon, and the cause needs to be ascertained. It should be remembered that cellulitis may occur concurrently with phlebitis or arterial insufficiency. Lyme disease with an erythema migrans rash without the typical “bull’s-eye” appearance may be mistaken for cellulitis; history of tick bite, macular appearance, and absence of tenderness help differentiate it from cellulitis. Acute allergic contact dermatitis may also mimic cellulitis or complicate other underlying conditions; lesions follow distribution of contact. Eosinophilic cellulitis (Wells syndrome) represents a recurrent hypersensitivity reaction (triggered by an insect bite, viral infection, drug, or vaccine and associated with peripheral blood eosinophilia) presenting on a limb as an area of localized erythema with sharp borders having a green/gray hue and progressing to indurated plaques that eventually resolve. Papular urticaria is seen mostly in young persons after an insect bite, with very itchy urticarial papules near the site of the bite that can coalesce into an indurated red plaque simulating cellulitis.
The diagnosis of cellulitis is primarily clinical.
History
Once it is established that cellulitis is present, predisposing factors should be identified. A history is obtained for diabetes, congestive heart failure, recent trauma, leg edema, claudication, previous infection, tinea pedis, and loss of sensation. One should ask about intravenous drug use, occupational exposure, and any recent bites and stings. A history of fever with rigors suggests bacteremia. Community outbreaks of MRSA skin infection should be checked into, as well as exposure to members of contact-sports teams and those attending day care facilities or persons recently hospitalized or institutionalized.
Physical Examination
Note is made of the temperature, area(s) of skin involved, lymphangitic streaking, proximal lymphadenopathy, heart murmur, peripheral edema, diminished peripheral pulses, decreased sensation, and any breaks in the skin, ulceration, or atrophy. Marking the borders of the lesion with an indelible pen allows objective and rapid assessment of progression and resolution. Crepitus or foul odor is suggestive of anaerobic infection. One palpates for fluctuance and inspects the viability of surrounding tissue. Any tinea, dermatitis, venous insufficiency, or previous injury should be noted. As noted, there are no distinguishing clinical features of MRSA-related cellulitis that differentiate it from non-MRSA disease.
Laboratory Studies
A complete blood cell count and differential are helpful in gauging the severity of infection and the hematologic response. Bacterial culture of the skin is indicated in patients who have open or weeping wounds, abscess, or infection in unusual areas, such as the perineum. Material from such areas should be cultured both anaerobically and aerobically and include testing for MRSA. Because most cases of uncomplicated cellulitis in immunocompetent persons are caused by streptococci or staphylococci, culturing is not routinely performed; however, with the rise in prevalence of community-acquired MRSA infection, culturing for MRSA is being urged, especially in the setting of skin abscess or wound. It can be difficult to culture the offending organism from unbroken skin; there is no evidence that aspiration from the advancing margin is superior to sampling from any other area of involved skin. Any associated abscess should be incised, drained, and cultured fully before initiation of antibiotic therapy (see Part 2 of this chapter).
When rigors, fever, heart murmur, or lymphangitic or deep tissue spread is present or the patient is immunocompromised, two separate sets of blood cultures should be obtained before any antibiotics are administered. Blood cultures are not routinely necessary for immunocompetent patients with localized cellulitis, but if there is a local outbreak of MRSA infection or MRSA is suspected on epidemiologic grounds (member of sports team, day care attendee, recent discharge from hospital, nursing home, or prison), blood cultures should be obtained. If crepitus, fluctuance, or devitalization is present, obtain a plain film to look for gas production in soft tissue, which is indicative of gangrene. In cases suggestive of adjacent osteomyelitis (especially in diabetic or immunocompromised patients), a plain film may be helpful, but if infection is too early to have caused obvious bony changes, a magnetic resonance imaging scan may be required.