TOPICS
2. The clinical signs and symptoms of AV disease
3. Physiologic compensatory mechanisms of AV disease
4. Echocardiography and AV disease
5. Surgical and catheter-mediated AV replacement
6. Anesthetic implications of AV diseases
7. Clinical scenario: The patient with mixed AS/AR and the emergency institution of CPB
The aortic valve (AV) is the gateway through which the stroke volume is ejected from the left ventricle (LV) into the systemic circulation. When the AV is normal, systolic and diastolic pressures are maintained and the delivery of oxygenated blood to the tissues unencumbered. Should the gateway be narrowed as in aortic stenosis (AS), the LV hypertrophies concentrically to generate increased pressure to push the blood through the reduced aortic valve orifice. The thickened heart muscle and the increased work of ventricular ejection augments the oxygen demand of the ventricle, which if not met, can result in the development of myocardial ischemia. On the other hand, if the valve is incompetent resulting in aortic insufficiency or regurgitation (AR), the left ventricle will dilate and hypertrophy eccentrically to accommodate the increased volume filling the LV cavity during diastole. Diastolic pressure falls, and coronary perfusion pressure (CPP) decreases, increasing the risk of ischemia. Providing anesthesia for patients with AV disease undergoing aortic valve surgery or noncardiac procedures can be challenging.
OVERVIEW
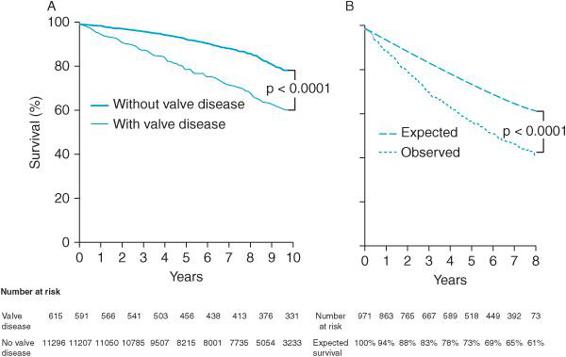
The American Heart Association Task Force on Practice Guidelines reports the current recommendations for the management of patients with AV disease.1 Although in the past valvular diseases were most likely the consequence of rheumatic heart disease, currently, in an increasingly aging population, degenerative diseases of the valves are most frequently diagnosed.2 More than one in eight individuals older than 75 years of age have moderate or severe valvular heart disease of one type or another.2 Life span is reduced in patients with severe valvular disease (Figures 6–1 and 6–2). Additionally, elderly women may have underdiag-nosed valvular heart diseases.2 Consequently, it is likely that as the population ages, AV disease will become ever more prevalent in cardiac surgical and noncardiac surgical patients alike. Sometimes, anesthesiologists are the first to diagnose a heart murmur during preoperative assessment.3 Van Klei et al in a study from the Netherlands found during routine preoperative examination a prevalence of 2.4% for AS in patients greater than 60 years of age scheduled for noncardiac surgery.3
THE CLINICAL SIGNS AND SYMPTOMS OF AV DISEASE
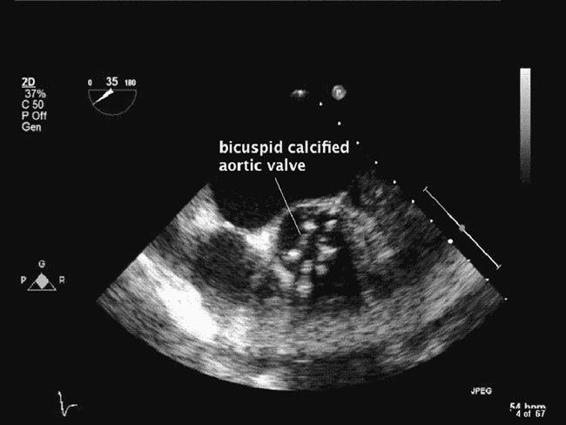
The aortic valve is normally tricuspid, with semilunar cusps and an aortic valve area (AVA) of 2.5 to 3.5 cm2. The most common causes of AS are degenerative changes of a normal trileaflet valve (Figure 6–3) or of a congenital bicuspid valve (Figure 6–4 and Videos 6–1 and 6–2).4 Other congenital malformations of the AV, such as unicuspid and quadricuspid valves, although uncommon, can lead to aortic stenosis even in the adolescent population. However, the majority of patients will develop AS as a consequence of inflammation, lipid accumulation, and calcification of normal trileaflet valves associated with aging.5 Degenerative processes also contribute to the development of AR.
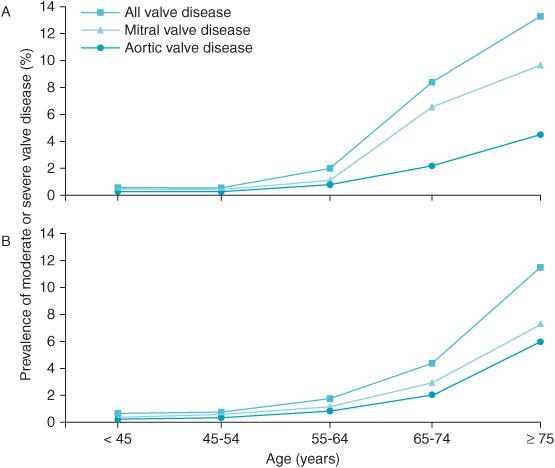
Figure 6–1. The increasing prevalence of valvular heart disease associated with age in population-based studies (A) and in one US county. (B) Anesthesiologists are increasingly likely to encounter patients with valvular disease as the population ages. (From: Nkomo VT, Gardin J, Skelton T, et al. Burden of valvular heart disease: a population based study. Lancet. 2006;368:1005-1011, with permission.)
Figure 6–2. Survival graphs after detection of moderate or severe valvular heart disease demonstrate decreased survival in population-based (A) and community (B) studies of patients with valvular heart disease. Graph A: survival in population-based studies. Graph B: expected versus observed survival in one US county of 971 residents diagnosed with valve disease between 1990 and 1995 compared with the expected survival of an age- and sex-matched population. (From: Nkomo VT, Gardin J, Skelton T, et al. Burden of valvular heart disease: a population-based study. Lancet. 2006;368:1005-1011, with permission.)
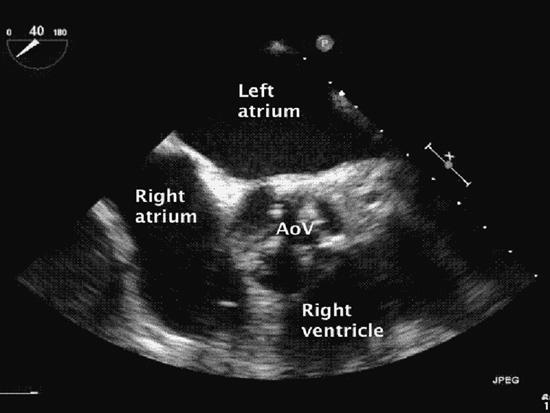
Figure 6–3. A calcified and stenotic aortic valve is seen in this midesophageal short-axis aortic valve view. Calcification of the aortic valve is usually associated with senile degeneration. However, congenitally abnormal (bicuspid) and rheumatic presentations also occur.
Figure 6–4. A congenitally bicuspid valve is seen in this midesophageal aortic valve short-axis view.
Aortic Stenosis
Severe AS is associated with an aortic valve area of less than 1.0 cm2. Calcification of the AV progresses with time which leads to the presentation of clinical symptoms including: angina, syncope, and dyspnea. With progressive stenosis, a pressure gradient develops across the valve and a systolic murmur results from the turbulent ejection of blood through the narrowed orifice. Over time, ventricular function can become impaired reducing the force of left ventricular ejection and surprisingly reducing the intensity of the murmur even as the stenosis itself remains unchanged or progresses. Patient survival following the onset of symptoms is fairly limited (2-5 y).6
Chronic Aortic Regurgitation
AR (Video 6–3) may occur as a consequence of any number of etiologies including: infectious endocarditis, rheumatic heart disease, connective tissue diseases producing aortic root dilatation, Marfan syndrome, vasculitis, and syphilis.1 Irrespective of the cause of AR the end result is the same. Namely, the leaflets of the aortic valve fail to coapt, allowing the retrograde flow of blood from the aorta into the left ventricular outflow track (LVOT) during diastole. The heart becomes volume overloaded from the incompetent valve, and develops eccentric hypertrophy and increased end-diastolic pressure.7 Patients frequently develop diastolic hypotension and a widened pulse pressure secondary to the run off of blood through the AV into the heart during diastole and the augmented ejection of blood volume during ventricular systole. Clinically, the patient with chronic AR frequently becomes dyspneic. Over time, the volume overloaded LV fails and heart failure ensues.
Acute Aortic Regurgitation
Unlike the patient with chronic AR, the acute AR patient often presents with pulmonary edema in the setting of acute congestive heart failure. The heart of the patient with chronic AR generally adapts to volume overload with time. The heart of the patient with acute AR is presented with a large, regurgitant volume challenge at the time of disease onset and does not have the opportunity to compensate. Such patients often present in cardiogenic shock. Conditions leading to acute AR include: aortic dissection, traumatic aortic injury, and endocarditis.
Combined Aortic Stenosis and Regurgitation
At times patients will manifest both AS and AR. Echocardiography can frequently help to discern the dominant physiologic derangement.
PHYSIOLOGIC COMPENSATORY MECHANISMS OF AV DISEASE
The heart of the patient with AV disease attempts to compensate for the additional pressure and/or volume work challenges presented to it by either an incompetent or a stenotic aortic valve.8
The LV of the patient with worsening AS attempts to produce ever-increasing ventricular systolic pressures in order to force blood through the narrowed aortic valve. Increased ventricular wall tension develops leading to an increased myocardial oxygen demand. Recall, LaPlace law:
Wall tension = (Ventricular pressur × Ventricular radius)/2 × Myocardial wall thickness
By increasing LV wall thickness, the heart compensates for the increased pressure necessary to overcome obstruction to systolic ejection. Increased wall thickness decreases wall tension. Although LV hypertrophy can over time compensate for worsening AS, compensatory adaptations have limits. Myocardial ischemia may develop secondary to inadequate blood supply to the thickened myocardium. The AS patient may also have coronary artery disease. Moreover, as wall tension increases and the heart becomes unable to compensate by increasing wall thickness myocardial oxygen demands increase even more. The thickened ventricle is also at risk for diastolic dysfunction because the hypertrophied LV is relatively noncom-pliant.9 Often the noncompliant ventricle tolerates volume challenges poorly and is heavily dependent upon atrial contraction to effectively load the LV during diastole. Particularly, the loss of the “atrial kick” through the development of atrial fibrillation greatly reduces the CO.
AR challenges the heart by overwhelming it with volume. Compensation in this instance is directed at increasing the heart’s ability to push an increased blood volume out of the LV.10 The heart attempts to dilate in order to accommodate ever greater amounts of diastolic volume. By dilating, over time, the heart can increase its diastolic capacity with minimal change in left ventricular end-diastolic pressure (LVEDP). Whereas the AS ventricle becomes noncompliant, the AR ventricle becomes increasingly compliant.1 The LV hypertrophies eccentrically to respond to both volume and pressure work challenges. The increased volume load of the LV produces an increase in stroke volume (SV) and can contribute to an increase in systolic pressure. Over time the LV can no longer dilate sufficiently to remain highly compliant. As LV compliance becomes impaired, the heart is no longer able to accommodate increased diastolic volume with a minimal increase in diastolic pressure. Intraventricular pressures increase leading to reduced CPP and increased wall tension. Heart failure ensues. As time passes, the heart no longer compensates for the challenges presented to it, resulting in subclinical ischemia, myocyte cell death, LV fibrosis, and ventricular dysfunction.8
The patient with acute AR lacks the time for any compensatory remodeling of the heart’s structure. In such instances, a rapid increase in left ventricular enddiastolic volume produces a dramatic rise in left ventricular end-diastolic pressure (LVEDP). The elevated LVEDP can result in myocardial ischemia and cardiogenic shock with systemic hypoperfusion and pulmonary edema. The anesthetic management of these patients can be very challenging as they frequently present to the operating room in cardiogenic shock.
ECHOCARDIOGRAPHY AND AV DISEASE
Basic views and diagnostic approaches to the AV were presented in the introduction to echocardiography at the start of this text and should be briefly reviewed before proceeding with this section. The role of echocardiography in the management of these patients cannot be underestimated.1
Aortic Stenosis
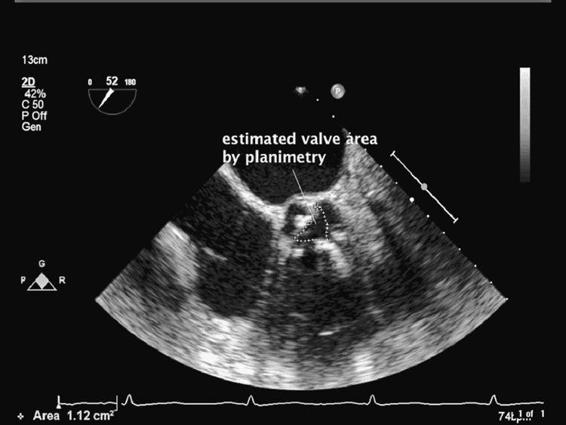
The aortic valve can be examined in the midesophageal short-axis (MESAX) and long-axis views (MELAX) of the aortic valve. The MESAX provides an ideal opportunity to examine the three leaflets of the valve. By using color flow Doppler turbulent flow across the AV can be seen. Planimetry (Figure 6–5) of the stenotic valve permits a rough estimate of the valve area. Planimetry of the AV is performed by obtaining the midesophageal short-axis view. Once the leaflets are imaged, the freeze button is pressed. Using the trace function the valvular orifice is highlighted. Estimates of AV orifice area by planimetry are at times unreliable secondary to poor visualization of the valve or secondary to leaflet thickening and calcification.11
Stenotic orifice area can be calculated using the “continuity equation” which follows the conservation of flow or mass. As water flows down a river its velocity is slow where the river is wide and its velocity is fast where it is narrow—the rapids!!! The flow of blood thorough the LVOT and the aortic valve follows the same conservation law.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree