FIGURE 17.1 Embolization of a giant cavernous aneurysm: Patient presented with severe retro-orbital headache and multiple cranial nerve palsies. A, B: Anterioposterior and lateral angiogram. C: Stent placement. The short arrows represent the stent markers and long arrow identifies the coiling microcatheter. D: Deployment of the stent across the base of the aneurysm. E, F: Six-month follow-up angiogram that shows stable and obliterated lesion. Patient had complete resolutions of symptoms.
The primary goals of anesthesia for INR procedures are to control the level of sedation in a manner that permits prompt neurologic examination, to render the patient immobile, and to manipulate cerebral hemodynamics. Many INR procedures such as diagnostic angiography, carotid stenting, and embolization of cerebral arteriovenous malformations (AVMs) can be undertaken with intravenous sedation. However, a growing number of INR procedures including coiling of intracranial aneurysms, intracranial angioplasty, and embolization of some high-flow AVMs require general anesthesia. General anesthesia is also a necessity for diagnostic procedures in children and in uncooperative adults as well as for prolonged procedures such as those on the spinal cord. Often the choice of anesthetic technique is a collaborative decision by the neurointerventionalist and the anesthesiologist, which is based on individual patient assessment.
Chapter Highlights
 The revised chapter builds on the strength of the previous version. It provides a general description of interventional radiologic procedures and discusses specific applications.
The revised chapter builds on the strength of the previous version. It provides a general description of interventional radiologic procedures and discusses specific applications.
 It reviews all relevant current literature.
It reviews all relevant current literature.
 It provides a new illustrative case report, figures, and tables.
It provides a new illustrative case report, figures, and tables.
 It provides an updated review of technical advances and pharmacologic agents and a description of new procedures.
It provides an updated review of technical advances and pharmacologic agents and a description of new procedures.
 It describes emerging applications such as thrombolysis, tumor treatment, and vertebroplasty.
It describes emerging applications such as thrombolysis, tumor treatment, and vertebroplasty.
 It includes updated information about embolic agents, sedatives, and anticoagulant drugs.
It includes updated information about embolic agents, sedatives, and anticoagulant drugs.
I. NEUROVASCULAR ACCESS AND METHODS
A. Vascular access. INR procedures typically involve insertion of catheters into the arterial circulation of the head or the neck, usually through the transfemoral route. As illustrated in Figure 17.2, transfemoral access is accomplished by the placement of a large introducer sheath into the femoral artery, usually 5.0 to 7.5 F in size. Through the introducer sheath, a 5.0 to 7.5 F coaxial catheter is positioned by fluoroscopic control into the carotid or vertebral artery. Finally, a 1.5 to 2.8 F superselective microcatheter is introduced into the cerebral circulation that can be used to deliver drugs, embolic substances, or balloons to distal regions of the brain. The transfemoral placement site is usually infiltrated with local anesthetic which can cause a femoral nerve block and temporary weakness of the quadriceps muscle. Transfemoral venous access can be used to reach the dural sinuses and in some cases the arterial side of the AVMs. Direct percutaneous puncture is used to access superficial lesions of the head and neck such as tumors and arteriovenous and venous malformations.
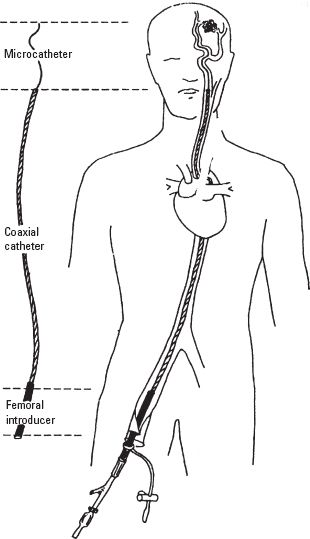
FIGURE 17.2 Representation of a typical arrangement of the transfemoral coaxial catheter system showing the femoral introducer sheath, the coaxial catheter, and the microcatheter. (From Young WL. Clinical Neuroscience Lectures. Munster, IL: Cathenart Publishing, 1999, with permission.)
B. Imaging technology. Radiologic imaging techniques needed for INR procedures include high-resolution fluoroscopy and high-speed digital subtraction angiography (DSA) with road mapping functions that enable the radiologist to observe the advance of the catheter against the background map of the patient’s cerebral vessels “in real time.” DSA involves the subtraction of images obtained before and after injection of radiocontrast drugs and facilitates visualization of only those vessels that are opacified by contrast injection. Any displacement of the cerebral vessels from movement of the head profoundly degrades DSA images. It is therefore critical that the patient remain immobile during the procedure.
Prolonged and sometimes repeated INR procedures carry the risk of radiation exposure to the patients and staff. Hence, considerable effort is being expended to design fluoroscopes that utilize less radiation and offer superior image quality. To achieve this, better x-ray tubes, x-ray generators, and spectral shaping filter technologies have been developed along with improved logic systems for automated dose rate and image quality control. The newer generation of fluoroscopes combines rotational angiography with computed tomography (CT). Digital images can be manipulated in three dimensions to investigate vascular geometry to optimize treatment.
C. Materials for embolization and infusion. Factors affecting the choice of embolic medium include the nature of the disease, the purpose of the embolization, the size and penetration of the emboli and vessels, and the permanency of the occlusion. The ideal choice and the combination of materials remain controversial. Embolic materials include balloons, coils, polyvinyl alcohol (PVA) particles, gelatinous embolization spheres, and n-butyl cyanoacrylate (NBCA) glue which is available as a liquid monomer that rapidly polymerizes in contact with ionic solutions such as blood and saline. Onyx is a non-adhesive liquid embolic system composed of ethylvinyl alcohol dissolved in dimethyl sulfoxide (DMSO). The use of non-adhesive liquid embolic materials decreases the chance of catheter impaction and permits the slower and more controlled delivery of embolic material. The potential systemic toxicity of DMSO can lead to pulmonary edema, bronchospasm, bradycardia, and cardiac arrest. An acute reduction in arterial saturation typically occurs 3 to 7 minutes after Onyx injection in about 10% to 30% of the cases. The desaturation lasts for 20 to 40 minutes and resolves without treatment. Patients may also complain of strong garlic taste during Onyx injection from the DMSO.
D. Radiation safety. There are three sources of radiation in the INR suite: direct radiation from the x-ray tube, leakage (through the collimator’s protective shielding), and scattered radiation (reflected from the patient and the area surrounding the body part to be imaged). The amount of exposure drops off proportionally to the square of the distance from the source of radiation (inverse square law). DSA delivers considerably more radiation than fluoroscopy. While working in the INR suite all persons should wear lead aprons, leaded glasses, and thyroid shields and have exposure badges.
II. ANESTHETIC CONSIDERATIONS
The primary responsibilities of the anesthesiologist in the INR suite are to provide the level of sedation/anesthesia that permits prompt neurologic assessment when needed; to render the patient physiologically stable and immobile; to manipulate the systemic blood pressure (BP) as dictated by the needs of the procedure; and to provide emergent care in the event of catastrophic complications.
A. Preoperative assessment. A careful assessment of the airway has to be made. A history of snoring may suggest that partial airway obstruction might occur with sedation. Snoring results in movement artifacts that may degrade the quality of images during cerebral angiography. The nature and severity of any adverse reaction to radiocontrast drugs need to be carefully assessed. The population of patients who have occlusive cerebrovascular disease may also require optimization of their treatment for coexisting conditions such as hypertension, heart failure, or angina. Preoperative communication with the INR team is essential to develop a clear strategy for sedation and hemodynamic interventions that might be needed during the procedure.
B. Preoperative investigations. Routine guidelines for laboratory investigations before surgery are applicable to INR procedures. Of particular interest is the baseline coagulation screen as anticoagulation will be required for the procedure.
C. Premedication. Anxiolytics may be administered depending on the condition of the patient. Minimal premedication is required for INR procedures. Oral nimodipine or transdermal nitroglycerin is sometimes used to decrease intraoperative vasospasm. Premedication with steroids and antihistaminics will also be required for patients who have a history of allergic reaction to radiocontrast drugs before exposure.
D. Room preparation. The INR suite should be equipped exactly as for delivering anesthesia in a standard operating room. Suction, gas evacuation, oxygen, and nitrous oxide should be available from the wall outlets. Ideally, the anesthesia machine should also be able to deliver carbon dioxide for deliberate hypercapnia. An extended anesthetic breathing system is necessary to reach the remotely located patient’s airway. Rapid access to all critical equipment should be possible at all times during the procedure. Induction and emergency drugs must be prepared for immediate use.
E. Patient positioning. Because INR procedures may last for several hours, it is essential that the patient be made as comfortable as possible before the start of sedation.
F. Intravenous access. During INR procedures the patients are often moved cephalad toward the image intensifier and away from the anesthesiologist so that the position of the catheters can be checked. This limits access to potential venipuncture sites and injection ports during the procedure. Therefore, adequate vascular access and sufficient tubing length should be in place before the start of the procedure. In adults, two intravenous cannulae are usually inserted for this purpose, one of which is at least 18 gauge in size. The infusions of anesthetic and vasoactive drugs should be in line before the patient is draped.
G. Monitoring
1. Arterial pressure. Because of the need to manipulate systemic hemodynamics and at times the emergent need for hemodynamic interventions, it is usually desirable to obtain direct systemic arterial pressure measurements during INR procedures. This is most conveniently achieved by transducing the side arm of the femoral introducer sheath (Fig. 17.2). If a relatively large coaxial catheter passes through the introducer, however, the arterial pressure trace will be “dampened.” Despite dampening, the mean arterial pressure (MAP) will still be reliable. To avoid excessive dampening of the femoral arterial tracing, the introducer sheath should be at least 0.5 F larger than the coaxial catheter. Radial artery cannulation may be desirable when systemic BP is to be monitored before insertion of the femoral introducer sheath such as during the induction of general anesthesia for coiling of intracranial aneurysms or when postoperative BP monitoring is necessary.
For a typical intracranial procedure, two other pressures may be measured in real time in addition to the systemic arterial pressure: the internal carotid or vertebral artery pressure through the coaxial catheter and the distal cerebral arterial pressure through either the microcatheter or a balloon-tipped catheter. The coaxial catheter pressure is monitored so as to detect any thrombus formation or vasospasm at the catheter tip as evidenced by a dampened arterial trace. High-volume heparinized flush solution is passed continuously through the coaxial tip to discourage thrombus formation. Hence, the arterial pressure reading characteristically increases by 10 to 20 mm Hg when recorded through the coaxial catheter.
The setup for measuring arterial pressures is shown in Figure 17.3. The pressure transducers and access stopcocks for blood withdrawal and zeroing are mounted, depending on the institutional preference, either on the sterile field or toward the anesthesiologist. Measurement of distal cerebral arterial pressures through the microcatheter is useful during embolization of AVMs (see Therapeutic Embolization of AVMs below). When a balloon catheter is used for internal carotid artery (ICA) occlusion, pressure measurements at the tip of the catheter provide the stump pressure (see Test Occlusion below).
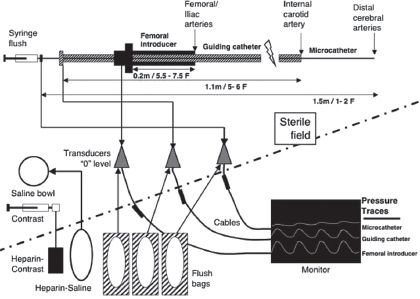
FIGURE 17.3 Schematic representation of pressure monitoring and the continuous flush systems in the interventional neuroradiology suite.
2. Other systemic monitoring includes five-lead electrocardiogram, preferably with ST segment trending and respiratory tracing, automated non- invasive BP, end-tidal carbon dioxide tension, and peripheral temperature monitors. Pulse oximeter probes are placed on the great toes and are useful for qualitatively comparing distal pulses in the lower limbs. Loss of the pulse oximeter tracing on the side of the femoral introducer sheath offers early warning of thromboembolism, vasospasm, or mechanical obstruction.
3. Central nervous system monitoring. During many procedures, neurologic examination provides adequate monitoring of central nervous system (CNS) integrity. Adjuncts that are especially useful during general anesthesia or planned proximal occlusion include electroencephalogram (EEG), somatosensory and motor evoked potentials, transcranial Doppler (TCD) ultrasound, and 133Xe cerebral blood flow (CBF) monitoring.
4. Urinary output. Most patients undergoing INR procedures will require bladder catheterization to assist in fluid management and to enhance patient comfort. Diuresis might occur during the procedure from the increase in intravascular volume as a result of continuous flushing of the intravascular catheters and the osmotic load from radiocontrast or mannitol injection. The timing and volume of contrast injected should be monitored, especially during prolonged procedures.
5. Laboratory tests. A baseline arterial blood gas (ABG) at the time of arterial puncture is useful to assess the gradient between arterial oxygen tension (PaO2) and arterial oxygen saturation (SaO2) as well as the arterial carbon dioxide tension (PaCO2)-to-end-tidal carbon dioxide tension (ETCO2) gradient. Activated clotting time (ACT) is used to monitor coagulation. The patients receive large quantities of fluid and radiocontrast and can diurese considerably so that a baseline hematocrit determination is helpful as well.
H. Anticoagulation. Careful management of coagulation is required to prevent thromboembolic complications from the presence of foreign bodies (catheters) and endothelial injury from the passage of microcatheters (Table 17.1). After placement of the femoral introducer sheath, a baseline ACT is obtained. Heparin, 2,000 to 5,000 U/70 kg, is given and another ACT obtained approximately 3 to 5 minutes later. The target ACT depends on the clinical needs and could be two to three times the baseline value. Additional heparin may be required throughout the procedure to maintain adequate anticoagulation. On occasion, when the INR procedure is completed, the anticoagulant effect of heparin is reversed with protamine and the femoral artery catheter is removed in the angiography suite. The proliferation of percutaneous closure devices has improved hemostasis at the arteriotomy site particularly in patients receiving thrombolytics and antiplatelet drugs.
TABLE 17.1 Anticoagulant Therapy
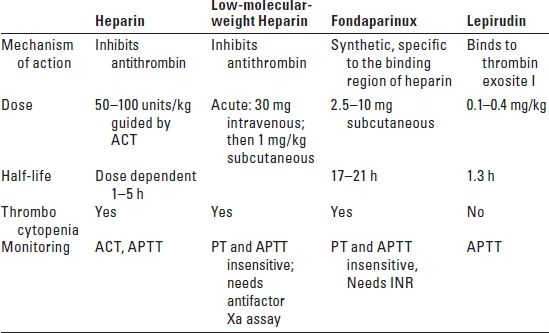
ACT, activated clotting time; APTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio.
III. DYNAMIC SEDATION
The selection of anesthetic drugs for conscious sedation is guided by the primary goals of alleviating pain, discomfort, and anxiety and ensuring patient immobility. At the same time, one must allow for a rapid decrease in the level of sedation when neurologic testing is required. The procedures are not generally painful except for sclerotherapy and chemotherapy. There is an element of pain associated with distention of or traction on the vessels; contrast injection into the carotid artery is frequently described as “burning.” Discomfort might result from prolonged periods of immobilization, bladder catheterization, and, to a lesser extent, the femoral puncture site. The procedure might also be psychologically stressful because of the potential risk of stroke during the procedure. Patient immobility, whether by conscious effort or deep sedation, is essential for INR procedures. Movement can not only degrade the quality of images but can also result in vascular injury. Patients who have impaired communication or dementia, sleep apnea, orthopnea, active congestive heart failure, unstable angina, anxiety, claustrophobia, or poor pain tolerance need to be carefully assessed regarding their suitability as candidates for dynamic sedation.
A. Sedation scores. Quantification of the level of sedation during an INR procedure is critical to the control of drug delivery. A variety of sedation scores are available. The Ramsay score that grades sedation at six levels is popular (Table 17.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






