CEREBRAL ANEURYSMS
 Epidemiology
Epidemiology
 Types of Aneurysms
Types of Aneurysms
 Pathophysiology
Pathophysiology
 Genetics and Associated Conditions
Genetics and Associated Conditions
 Clinical Presentation and Grading
Clinical Presentation and Grading
 Diagnosis of Cerebral Aneurysms
Diagnosis of Cerebral Aneurysms
 SUBARACHNOID HEMORRHAGE (SAH)
SUBARACHNOID HEMORRHAGE (SAH)
 COMPLICATIONS OF SUBARACHNOID HEMORRHAGE
COMPLICATIONS OF SUBARACHNOID HEMORRHAGE
 Rebleeding
Rebleeding
 Vasospasm
Vasospasm
 Central Nervous System Complications
Central Nervous System Complications
1. Hydrocephalus
2. Seizures
 Systemic Sequelae
Systemic Sequelae
1. Fluid and electrolyte disturbance
2. Cardiac abnormalities
3. Pulmonary abnormalities
4. Other medical complications
 OPERATIVE ANEURYSMAL OBLITERATION
OPERATIVE ANEURYSMAL OBLITERATION
 Anesthesia for Aneurysm Surgery
Anesthesia for Aneurysm Surgery
 Temporary Proximal Occlusion
Temporary Proximal Occlusion
 Intraoperative Aneurysmal Rupture
Intraoperative Aneurysmal Rupture
 ENDOVASCULAR TREATMENT
ENDOVASCULAR TREATMENT
 ARTERIOVENOUS MALFORMATIONS (AVMs) OF THE BRAIN
ARTERIOVENOUS MALFORMATIONS (AVMs) OF THE BRAIN
 Definition
Definition
 Presenting Signs and Symptoms
Presenting Signs and Symptoms
 Neurologic Damage
Neurologic Damage
 Unfavorable Prognostic Features
Unfavorable Prognostic Features
 Imaging
Imaging
 Treatment
Treatment
 Management of Associated Aneurysms
Management of Associated Aneurysms
 Anesthetic Management
Anesthetic Management
 Cerebral Circulatory Changes
Cerebral Circulatory Changes
 AVMs and Pregnancy
AVMs and Pregnancy
 Vein of Galen AVMs
Vein of Galen AVMs
I. CEREBRAL ANEURYSMS
The anesthetic and perioperative management of the surgical and the endovascular treatment of intracranial aneurysms, which are abnormal focal dilatations of cerebral arteries usually located at branch points, is designed to facilitate the conduct of the procedure and the patient’s recovery. The goal of management is to improve functional survival while minimizing the risk of aneurysmal rupture, cerebral ischemia, neurologic deficit, and associated systemic morbidity.
A. Epidemiology
Cerebral aneurysms have been reported to occur in every racial group in the world with an age range from 7 to 70 years. The size of an intracranial aneurysm is directly correlated with the risk of rupture and also of treatment. The annual incidence of aneurysmal subarachnoid hemorrhage (SAH) is approximately 6 to 8/100,000 in most western populations. The rate of rupture of an intracranial aneurysm is 0.05% to 6% per year, depending on the size and the location. Aneurysms are 11 times more likely to rupture in patients who have had a previous SAH than in those who have an asymptomatic aneurysm. Smoking and hypertension are risk factors. The incidence in men outnumbers women until age 50; women predominate thereafter. Aneurysms commonly present in the sixth decade of life. Approximately 20% of patients have more than one aneurysm. Aneurysmal SAH accounts for 10% of all cerebrovascular accidents.
B. Types of aneurysms
1. Saccular aneurysms are < 2.5 cm in diameter and are by far the most common. These are formed by the disintegration of the elastic layer of the artery at the flow separator region due to the pressure of the arterial pulse wave.
2. Giant aneurysms are up to 10 cm in diameter and represent 5% of all aneurysms.
3. Other types of aneurysms include dissecting (from a tear in the luminal endothelium that permits a blood column to dissect between the endothelium and the media), fusiform (associated with severe atherosclerosis or degenerative processes in childhood), traumatic (develop within 2 to 3 weeks after severe head injury), dolichoectatic, mycotic (infectious), and serpentine (with or without internal thrombosis).
C. Location
Ninety percent of aneurysms occur on the anterior circulation, most commonly the internal carotid-posterior communicating artery (more common in women), anterior communicating artery (more common in men), and middle cerebral artery (MCA) bifurcation. Ten percent occur on the posterior circulation, most commonly at the basilar apex. The internal carotid artery is affected in children. Complication rates are reported to be highest for anterior communicating artery (ACA) aneurysms in the anterior circulation and aneurysms of the basilar bifurcation in the posterior circulation.
D. Natural history
One patient in six will die within minutes of an SAH. Of the patients who survive to be admitted to the hospital, 25% will die thereafter, and just over 50% will recover partially or completely. Without treatment, at least 50% of ruptured aneurysms will rerupture within 6 months and then at a rate of 3% per year.
E. Prognostic factors
The rate and the volume of bleeding affect the patient’s neurologic condition at hospital admission and determine outcome. Patients who remain conscious and complain only of severe headache after SAH do better than patients who are comatose upon arrival at the hospital. Older age, poor general health, evidence of clots in either the brain substance or the ventricles, and repeat hemorrhage all affect outcome adversely (Table 10.1).
TABLE 10.1 Predictors of Mortality after Subarachnoid Hemorrhage

F. Genetics and associated diseases
Of patients who have SAH, 5% to 10% will have one or more first-order relatives who have also had a ruptured aneurysm. The inheritance is probably dominant with variable penetrance. Conditions associated with intracranial aneurysms include polycystic kidney disease (5% of aneurysms series, 33% of polycystic kidney series), coarctation of the aorta (1% of aneurysm patients, 5% of coarctation patients, all of whom are hypertensive), sickle cell disease, drug abuse (cocaine: generalized vasoconstriction and hypertension; intravenous use: mycotic aneurysms), and hypertension (30% to 40% of patients who have SAH). More rarely associated conditions include fibromuscular dysplasia, Marfan’s syndrome, tuberous sclerosis, Ehlers-Danlos syndrome, hereditary hemorrhagic telangiectasia, moyamoya disease, and pseudoxanthoma elasticum. Choriocarcinoma and cardiac myxomas are associated with multiple cerebral aneurysms.
The cost-effectiveness of screening asymptomatic individuals, even when there is a familial history of intracranial aneurysms, has not been demonstrated so that screening with magnetic resonance angiography (MRA) combined with computed tomography angiography (CTA) should be considered on a case-by-case basis although catheter angiography remains the gold standard for aneurysm detection.
II. SUBARACHNOID HEMORRHAGE
Most intracranial aneurysms remain asymptomatic until they rupture. Aneurysmal SAH from rupture of an intracranial aneurysm is reported to occur during physical exertion or stress but can happen at any time. Risk factors for SAH include hypertension, smoking, alcoholism, sympathomimetic drug use, polycystic kidney disease, Ehlors-Danlos syndrome, and a family history of SAH (Table 10.2).
TABLE 10.2 Risk Factors for Aneurysmal SAH

SAH, subarachnoid hemorrhage.
A. The clinical presentation of patients after aneurysmal SAH is classic, with 80% of awake patients complaining of a severe throbbing headache which they describe as “the worst headache of my life.” A warning or a sentinel headache is also described by approximately 20% of patients. The headache may be accompanied by malaise, nausea and vomiting, and neck stiffness. This “warning leak” or “sentinel bleed” is reported to precede a major SAH in approximately 40% of patients (range: 5% to 70%). Approximately half of these patients seek medical attention before their major SAH, and 16% to 60% of them are misdiagnosed. Common misdiagnoses include flu, meningitis, cervical disc disease, migraine, cluster headaches, myocardial infarction, malingering, and intoxication. This is of significance because the overall clinical course and outcome are worse in misdiagnosed patients. Misdiagnosis was reported to be associated with a nearly fourfold greater likelihood of death or disability at 1 year in patients who had minimal or no neurologic deficit at the initial visit. More serious sequelae of SAH include change in mental status (combativeness, irritability) and level of consciousness, collapse, seizures, cranial nerve palsy, and intracranial hypertension from mass effect.
B. SAH is a true medical emergency. Rupture of an intracranial aneurysm accounts for approximately 80% of cases with a high rate of complications and death. An estimated 10% to 15% of patients die before reaching the hospital. The 30-day mortality rate approaches 45% and approximately half of the patients die within the first 6 months. Mortality and morbidity increase with age and poorer overall health and more than half the survivors have major neurologic deficits. Physical examination and laboratory studies identify changes in level of consciousness, focal neurologic deficits, intracranial hypertension, fever, meningismus, nuchal rigidity (may be absent early after SAH), photophobia, ophthalmic hemorrhages (poor prognostic sign), and fluid (hypovolemia) and electrolyte (hyponatremia) imbalance.
C. Grading. In 1956, Botterell introduced a system for grading patients after SAH to facilitate assessment of surgical risk, prediction of outcome, and prompt evaluation of the patient’s condition. Grades I to V describe the patient’s level of consciousness and degree of neurologic impairment, with each higher grade representing greater severity. Hunt and Hess modified Botterell’s system in 1968 to include a provision for the effect of serious systemic illness (Table 10.3A).
TABLE 10.3A Clinical Grading after Subarachnoid Hemorrhage: Hunt and Hess Modification
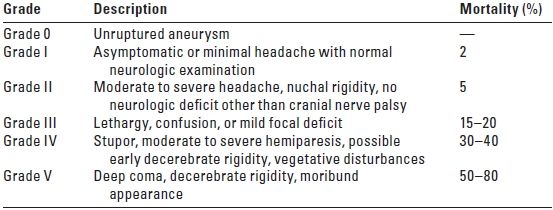
Serious systemic diseases (hypertension, coronary artery disease, chronic pulmonary disease, diabetes) and severe vasospasm on angiography cause assignment of the patient to the next less favorable category. From Hunt WE, Hess EM. J Neurosurg. 1968;28:14, with permission.
The World Federation of Neurological Surgeons (WFNS) 1988 grading scale, based on the Glasgow Coma Scale, demonstrated that the preoperative level of consciousness correlated most directly with outcome (Table 10.3B).
TABLE 10.3B World Federation of Neurological Surgeons Clinical Grading Scale

WFNS, World Federation of Neurological Surgeons; GCS, Glasgow Coma Scale. From Drake CG, Hunt WE, Kassell N, et al. J Neurosurg. 1988;68:985, with permission.
D. Diagnosis of cerebral aneurysms
1. Computed tomography (CT) and angiography (CTA): A non-contrast cranial CT scan to assess the amount of subarachnoid, intracerebral, and intraventricular blood is the first step in the diagnosis of SAH. The probability of detecting hemorrhage is proportional to the timing and the clinical grade of the bleed. The sensitivity of the CT scan for identifying SAH is 98% to 100% in the first 12 hours after SAH. This declines in the next 24 hours to 93% and then to 57% to 85% in the 6 days after the initial SAH.
CTA is a non-invasive, rapid, and readily available modality that is fairly sensitive for large-size cerebral aneurysms. Although effective in diagnosing severe vasospasm, CTA is less accurate in detecting moderate and mild vasospasm and not useful at all in patients who have already had clipping or coiling of an aneurysm owing to artifacts from the metal in aneurysm clips and coils.
When combined with catheter angiography, CTA is able to define intraluminal aneurysmal thrombosis and demonstrate calcification of the aneurysmal wall and its relation to intracerebral hemorrhage and the bony landmarks. The sensitivity and the specificity of CTA for aneurysmal detection depend on the quality of the images obtained, expertise of the radiologist, and size and location of aneurysms. The reported sensitivity of CTA for aneurysms is 70% to 100%; the specificity is 79% to 100%. When aneurysmal size is considered, CTA sensitivity for aneurysms ≥ 5 mm is 95% to 100%; for aneurysms < 5 mm, the sensitivity is 64% to 83%. Both the sensitivity and the specificity of CTA for aneurysm diagnosis increase with the experience of the radiologist. CTA is considered equal to catheter angiography in 80% to 83% of cases while in 74% of patients, catheter angiography performed after CTA did not add any new information. Thus, when the aneurysm is demonstrated on CTA, many neurosurgeons opt to operate on the basis of CTA alone in cases where the risk of delaying surgery for catheter angiography is too great.
2. Lumbar puncture is recommended for diagnostic analysis of cerebrospinal fluid (CSF) if the CT scan is negative as the sensitivity of the CT scan is not 100% even in the first few hours after SAH. Technique, timing, and interpretation of the CSF sample are vital to the accurate diagnosis of SAH. The sample is studied for red blood cells, white blood cells, xanthochromia, and the presence of bilirubin. A “warning leak” can be excluded and a favorable outcome expected if both CT and CSF results are negative after a history of severe headache.
3. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) demonstrate the true size of the aneurysm, its relation to important anatomic structures, the size, shape, and amount of clot in the lumen of aneurysm, and any recent or old hematoma. MRI and MRA images may also indicate other possible causes of SAH in patients who have a negative CT scan and an equivocal CSF examination.
MRA does not require iodinated contrast and ionizing radiation and thus is useful in evaluating pregnant patients. MRA is not, however, sufficiently sensitive for detecting small aneurysms. The sensitivity of MRA with aneurysms ≥ 5 mm is 85% to 100%; this decreases to 56% for aneurysms < 5 mm in size. The sensitivity of 3-dimensional (3D) timeof-flight MRA for cerebral aneurysms is between 55% and 93%.
4. Cerebral angiography, although invasive, still remains the gold standard for the diagnosis of a cerebral aneurysm as the cause of SAH. This modality gives the exact location, size, and configuration of the aneurysm with good visualization of the neck of the aneurysm.
III. COMPLICATIONS OF ANEURYSMAL SAH (TABLE 10.4)
TABLE 10.4 Complications of Aneurysmal Subarachnoid Hemorrhage
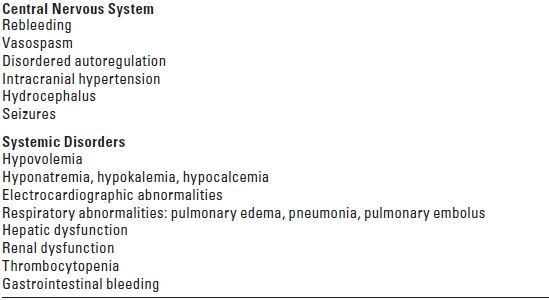
A. Rebleeding after SAH
The occurrence of further hemorrhage after the initial SAH is one of the major causes of neurologic deterioration after SAH. The size of the hematoma from the episode of rebleeding is the most critical factor in determining outcome. Patients who have a large subdural hematoma and a marked midline shift on CT scan have a poorer prognosis, as do those who have associated intracerebral and intraventricular hemorrhage (See Table 10.5).
TABLE 10.5 Factors Predisposing to Rebleeding after SAH

SAH, subarachnoid hemorrhage.
1. The cardinal signs are deterioration in the level of consciousness, development of focal neurologic deficits (aphasia, hemiplegia), abnormal vital signs (hypertension, bradycardia, arrhythmias, irregular respirations), and the presence of hemorrhage on ophthalmic examination.
These subsequent episodes of aneurysmal rupture are usually more severe than the first hemorrhage. The mortality associated with a second hemorrhage rises precipitously with significant morbidity in the surviving patients. Late rebleeding is fatal in 67% of cases. All rebleeding accounts for 22% of the mortality from SAH. Several factors predispose to rebleeding (Table 10.5). Increasing admission Hunt-Hess grade and aneurysm size are independent predictors of rebleeding.
2. The incidence of rebleeding is highest (4%) during the first 24 hours after SAH and then declines to 1% to 2% per day for the next 13 days. Approximately 20% to 30% of ruptured aneurysms rebleed within the first 30 days after SAH. The cumulative risk of rebleeding is 19% at 2 weeks, 50% at 6 months, and then decreases to 3% per year for up to 15 years. During the ensuing 5 months after the initial SAH, 10% to 15% of patients will rebleed. The overall incidence of rebleeding is 11%. The incidence of aneurysmal rupture during induction of anesthesia is 0.5% to 2% and carries a mortality of 75%. The incidence of intraoperative aneurysmal rupture varies from 6% to 18%, depending on the institution and the size and the location of the aneurysm. In order of decreasing incidence, the causes of rupture during operation include aneurysmal dissection, brain retraction, hematoma evacuation, and dural and arachnoid opening.
3. Pathophysiologic sequelae of rebleeding include intracranial hypertension and compromised cerebral perfusion; acute hydrocephalus from the sudden deposition of subarachnoid clot that obstructs the flow of CSF through the basal cisterns; cerebral infarction from either direct, hematoma-induced destruction of tissue or shifts in the intracranial contents with vascular compromise; impaired autoregulation (the ability of the normal brain to maintain cerebral blood flow [CBF] at a fairly constant level between a mean arterial pressure [MAP] of 50 and 150 mm Hg); and a reduction in the cerebral metabolic rate for oxygen consumption (CMRO2).
4. Prevention of rebleeding (Table 10.6) is only accomplished definitively by early surgical or endovascular obliteration of the aneurysm. Either operation or endovascular obliteration within 24 to 48 hours of SAH is, therefore, favored because of the association with improved outcome. At 1-year follow-up, the results of the International Subarachnoid Aneurysm Trial comparing operative aneurysmal clip ligation with endovascular metal coiling demonstrated that the risk of rebleeding was low in both groups: 1% for the surgical group and 2.4% for the endovascular group. The effects of rebleeding were taken into account in determining that the relative risk of either death or significant disability for endovascular patients is 22.6% lower than for surgical patients. This represented an absolute risk reduction of 6.9%. Most of the 2,143 patients randomized into the trial were in good condition (WFNS Grades I and II) after SAH and had small anterior-circulation aneurysms (92% < 11 mm in size) for which both endovascular coiling and neurosurgical clipping were considered therapeutic options.
TABLE 10.6 Prevention of Rebleeding after SAH

SAH, subarachnoid hemorrhage; ICP, intracranial pressure.
All protocols designed for prevention of rebleeding after SAH include bedrest as part of the therapeutic package. Control of systolic hypertension and prevention of increases in the transmural pressure gradient (TMPG) across the wall of the aneurysm (MAP minus intracranial pressure [ICP]) are recommended. This can be achieved by the intravenous infusion of short-acting drugs (e.g., esmolol, labetalol), which have a good safety profile and a reliable dose–response relationship, to control either labile hypertension or transient spikes in blood pressure from therapeutic interventions. The routine use of nimodipine as prophylaxis against vasospasm needs to be taken into consideration as it also contributes to the lowering of systemic blood pressure.
It is equally important to avoid hypotension and to maintain the patient’s “normal” blood pressure as the lower acceptable limit as means of preventing either initiation or exacerbation of vasospasm from a decrease in cerebral perfusion pressure (CPP), the difference between MAP and ICP. The use of narcotic analgesics and sedatives is recommended in titrated doses to reduce pain and anxiety while avoiding oversedation, hypoventilation, and hypercarbia with a consequent rise in ICP.
Avoiding hypovolemia and ensuring normovolemia are crucial to the successful treatment of hypertension and the prevention of hypo-tension, vasospasm, hypoperfusion, and the consequent development of cerebral ischemia, infarction, and new neurologic deficit. Control of any seizure activity is also vital as seizures may themselves lead to hypertension and an increase in the cerebral oxygen requirement.
Control of ICP is important in poor-grade patients who may have intracranial hypertension after SAH. The rapid drainage of cerebrospinal fluid (CSF) from either lumbar or ventricular puncture is avoided, however, as this may lead to a deleterious fall in ICP with a relative rise in transmural pressure and the potential for aneurysmal rerupture. When cerebral perfusion is seriously compromised because of intracranial hypertension from intracranial hematoma or cerebral swelling, CSF drainage may be instituted to reduce ICP, along with other strategies.
The use of antifibrinolytic drugs such as tranexamic acid and epsilon–aminocaproic acid has been shown to reduce the rate of rebleeding in the initial 2 weeks after SAH. There was, however, an increase in the incidence of cerebral ischemia and infarction that was shown to offset any improvement in overall outcome in the older studies. Recent studies, however, have demonstrated a reduction in the rate of early rebleeding and adverse outcome when tranexamic acid is given immediately after making the diagnosis of SAH. Newer recommendations thus include the early administration of a short course of antifibrinolytic therapy in combination with early treatment of the aneurysm by either endovascular obliteration or craniotomy for clip ligation, depending on the clinical situation. This mandates the rapid and efficient accomplishment of diagnosis, evaluation, and initial treatment. Administration of the antifibrinolytic drug is discontinued after the aneurysm is secured and prophylaxis against hypovolemia and vasospasm is instituted. Antifibrinolytic therapy is also recommended in patients who are at low risk of vasospasm and for whom there may be a beneficial effect from delaying surgery.
5. Management of rebleeding after SAH is designed to maintain CPP, limit intracranial hypertension, decrease intracranial volume, control systemic blood pressure, reduce transmural pressure across the wall of the aneurysm, and maintain cerebral oxygen delivery through normal arterial oxygen saturation and hemoglobin concentration.
B. Vasospasm or delayed ischemic deficit
Vasospasm is the reactive narrowing of the larger conducting arteries in the subarachnoid space that are surrounded by clots after SAH and affected by spasmogenic breakdown products of the red blood cells within the clot. The subsequent delayed ischemic deficit and infarction caused by vasospasm are major causes of disability and death after SAH, accounting for 30% of SAH-induced morbidity and mortality. Vasospasm has been considered the causative factor in 28% of all deaths and 39% of all disability after SAH and is, therefore, responsible for the great human cost and extensive utilization of limited health care resources.
Patients of all neurologic grades have a 50:50 chance of developing significant angiographic vasospasm. Symptoms of delayed ischemia occur in 20% to 25% of patients, and 30% to 50% of patients have evidence of infarction from vasospasm on CT scan. Death from vasospastic infarction occurs in 5% to 17% of patients.
The incidence of vasospasm peaks between the fourth and the ninth day after SAH and decreases over the next 2 to 3 weeks.
Vasospasm is directly related to the severity of the hemorrhage from the aneurysmal rupture, which correlates well with the location and the volume of blood noted on the post-SAH CT scan. The risk of vasospasm is increased by SAH-induced cerebral dysautoregulation and abnormal carbon dioxide (CO2) responsiveness, a Glasgow Coma Scale score of < 14 on hospital admission, an early increase in mean MCA flow velocity on transcranial Doppler (TCD) ultrasonography, and anterior cerebral and internal carotid artery aneurysms. The timing of surgery and the method of occlusion—surgical versus endovascular—have no effect on the subsequent development of vasospasm. The intraoperative transfusion of packed red blood cells is a risk factor for poor outcome, and postoperative transfusion is correlated with the development of angiographically-confirmed vasospasm. The mechanism may involve either the depletion or the inactivation of nitric oxide, an endogenous vasodilator, which transfused red cells lack.
1. Diagnosis of vasospasm
a. Clinical signs include either progressive impairment in the level of consciousness or the appearance of new focal neurologic deficits > 4 days after the initial SAH that are not associated with any other structural or metabolic cause. The onset may be either sudden or insidious and accompanied by increased headache, meningismus, and fever.
b. TCD ultrasonography may be used to determine the efficacy and the duration of treatment. Both a large increase in blood flow velocity (MCA velocity > 120 cm/second) and a rapid rise in blood flow velocity (> 50 cm/second in 24 hours) reflect a reduction in vessel caliber. A peak flow velocity of 140 to 200 cm/second indicates moderate vasospasm; a peak flow velocity > 200 cm/second is associated with severe vasospasm. Critically high blood flow velocities (>120 cm/ second) correlate strongly with vasospasm on angiography. Because TCD is operator dependent and reflects technical factors, ICP, cardiac output, and the artery being assessed, it is important to correlate TCD results with sequential neurologic examination and ICP, blood pressure, and cardiac output. In addition, absolute values for TCD can be misleading in the setting of “Triple-H” therapy (hypertension/hypervolemia/hemodilution). The Linegaard ratio (ratio of the velocity in the vessel in question to the velocity in the ipsilateral internal carotid artery) has been shown to be helpful in following the TCD trends and as a guide to therapy.
c. Cerebral angiography is the most reliable modality for diagnosing and evaluating vasospasm. Although some angiographic evidence of vasospasm occurs in 70% to 80% of cases, only one-third of patients develop the clinical picture. Signs and symptoms of decreased CBF usually occur when the reduction in the diameter of the arterial lumen exceeds 50%, the definition of angiographically-severe vasospasm.
d. Xenon-enhanced CT, a relatively inexpensive technique, demonstrates the decrease in regional cerebral blood flow (rCBF) in patients who have clinical vasospasm. This technique can quantify rCBF accurately, be repeated within 20 minutes, fuse rCBF data with conventional CT scan anatomy, and distinguish ischemia from other causes of neurologic deterioration after SAH.
e. Jugular bulb oximetry detects changes in cerebral oxygen extraction (AVDO2). Patients who develop clinical vasospasm have a significant rise in AVDO2 approximately 1 day before the onset of signs of neurologic deficit. Increases in AVDO2 may therefore predict impending clinical vasospasm, while an improvement in AVDO2 reflects the patient’s response to treatment.
f. CBF-measuring modalities include positron emission tomography, which demonstrates a fall in CMRO2 after SAH, and single photon emission computed tomography (SPECT). Angiographic vasospasm, delayed ischemic deficit, and increased TCD velocities are associated with regions of hypoperfusion on SPECT.
2. Treatment of vasospasm involves pharmacologic and mechanical modalities.
a. Early operation for clip ligation of the aneurysmal neck permits the removal of a fresh clot by irrigation and suction. The surgeon may instill recombinant tissue plasminogen activator (rtPA) directly into the subarachnoid space to dissolve the remaining clot. This fibrinolytic drug can reduce vasospasm, but it also may cause bleeding by dissolving normal clots. Therefore, only patients at great risk of developing clinically-significant vasospasm are candidates for this treatment.
b. Timely securing of the aneurysm facilitates the subsequent treatment of vasospasm with hemodynamic and pharmacologic therapies. While better-grade patients (WFNS Grades I to III on hospital admission) whose aneurysms were occluded endovascularly were less likely to develop symptomatic vasospasm as compared with those undergoing surgical clip ligation, there was no significant difference in overall outcome between the two groups at the longest follow-up period.
c. The prophylactic use of the calcium antagonist nimodipine within 96 hours of SAH is now a standard aspect of care after SAH. Although nimodipine reduces the incidence of vasospasm, the improvement in mortality has not been statistically significant when compared with control groups. Because nimodipine tends to decrease blood pressure, patients may require hydration and the administration of pressor drugs during the induction of anesthesia and careful attention to fluid balance intra- and postoperatively.
d. Enoxaparin, a low-molecular-weight heparin given as one subcutaneous injection of 20 mg/day, has been shown to improve overall outcome at 1 year after SAH by reducing delayed ischemic deficit and cerebral infarction. Patients receiving enoxaparin also had fewer intracranial bleeding events and a lower incidence of severe shunt-dependent hydrocephalus.
e. Other drugs used to treat vasospasm include tirilazad, an antioxidant and free-radical scavenger whose clinical trials have demonstrated mixed results; nicaraven, a free-radical scavenger associated with a trend toward improved mortality, good outcome, and smaller infarct size at 3 months; ebselen, an antioxidant and anti-inflammatory drug whose neuroprotective properties have caused it to be effective in the treatment of acute stroke; and fasudil, a kinase inhibitor used intra-arterially to treat vasospasm. The use of endothelin antagonists has been associated with an increase in the incidence of pneumonia and hypotensive episodes. Preliminary studies have suggested that statins (e.g., simvastatin and pravastatin) may reduce vasospasm and improve mortality.
f. “Triple-H therapy,”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






